当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
QM/MM Studies of Dph5 – A Promiscuous Methyltransferase in the Eukaryotic Biosynthetic Pathway of Diphthamide
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1021/acs.jcim.8b00217 Johanna Hörberg 1 , Patricia Saenz-Mendez 2 , Leif A. Eriksson 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1021/acs.jcim.8b00217 Johanna Hörberg 1 , Patricia Saenz-Mendez 2 , Leif A. Eriksson 1
Affiliation
|
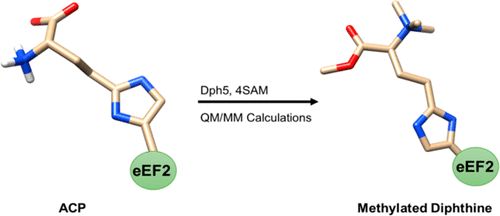
Eukaryotic diphthine synthase, Dph5, is a promiscuous methyltransferase that catalyzes an extraordinary N,O-tetramethylation of 2-(3-carboxy-3-aminopropyl)-l-histidine (ACP) to yield diphthine methyl ester (DTM). These are intermediates in the biosynthesis of the post-translationally modified histidine residue diphthamide (DTA), a unique and essential residue part of the eukaryotic elongation factor 2 (eEF2). Herein, the promiscuity of Saccharomyces cerevisiae Dph5 has been studied with in silico approaches, including homology modeling to provide the structure of Dph5, protein–protein docking and molecular dynamics to construct the Dph5-eEF2 complex, and quantum mechanics/molecular mechanics (QM/MM) calculations to outline a plausible mechanism. The calculations show that the methylation of ACP follows a typical SN2 mechanism, initiating with a complete methylation (trimethylation) at the N-position, followed by the single O-methylation. For each of the three N-methylation reactions, our calculations support a stepwise mechanism, which first involve proton transfer through a bridging water to a conserved aspartate residue D165, followed by a methyl transfer. Once fully methylated, the trimethyl amino group forms a weak electrostatic interaction with D165, which allows the carboxylate group of diphthine to attain the right orientation for the final methylation step to be accomplished.
中文翻译:

Qph / MM研究Dph5 –白喉酰胺的真核生物合成途径中的甲基转移酶
真核白氨酸合酶Dph5是一种混杂的甲基转移酶,可催化2-(3-羧基-3-氨基丙基)-1-组氨酸(ACP)的非常规N,O-四甲基化反应生成白氨酸甲酯(DTM)。这些是翻译后修饰的组氨酸残基二乙酰胺(DTA)(真核生物延伸因子2(eEF2)的独特且必不可少的残基部分)的生物合成中间体。在本文中,已通过计算机研究了酿酒酵母Dph5的混杂性这些方法包括提供Dph5结构的同源性建模,构建Dph5-eEF2复合物的蛋白质-蛋白质对接和分子动力学,以及概述合理机制的量子力学/分子力学(QM / MM)计算。计算表明,ACP的甲基化遵循典型的S N 2机理,首先在N-位发生完全甲基化(三甲基化),然后是一次O-甲基化。对于三个N中的每个-甲基化反应,我们的计算支持一个逐步的机制,该机制首先涉及质子通过桥联水转移至保守的天冬氨酸残基D165,然后进行甲基转移。一旦完全甲基化,三甲基氨基就会与D165形成弱的静电相互作用,从而使白氨酸的羧酸酯基获得正确的方向以完成最终的甲基化步骤。
更新日期:2018-06-21
中文翻译:

Qph / MM研究Dph5 –白喉酰胺的真核生物合成途径中的甲基转移酶
真核白氨酸合酶Dph5是一种混杂的甲基转移酶,可催化2-(3-羧基-3-氨基丙基)-1-组氨酸(ACP)的非常规N,O-四甲基化反应生成白氨酸甲酯(DTM)。这些是翻译后修饰的组氨酸残基二乙酰胺(DTA)(真核生物延伸因子2(eEF2)的独特且必不可少的残基部分)的生物合成中间体。在本文中,已通过计算机研究了酿酒酵母Dph5的混杂性这些方法包括提供Dph5结构的同源性建模,构建Dph5-eEF2复合物的蛋白质-蛋白质对接和分子动力学,以及概述合理机制的量子力学/分子力学(QM / MM)计算。计算表明,ACP的甲基化遵循典型的S N 2机理,首先在N-位发生完全甲基化(三甲基化),然后是一次O-甲基化。对于三个N中的每个-甲基化反应,我们的计算支持一个逐步的机制,该机制首先涉及质子通过桥联水转移至保守的天冬氨酸残基D165,然后进行甲基转移。一旦完全甲基化,三甲基氨基就会与D165形成弱的静电相互作用,从而使白氨酸的羧酸酯基获得正确的方向以完成最终的甲基化步骤。



























 京公网安备 11010802027423号
京公网安备 11010802027423号