当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A new twist on sea silk: the peculiar protein ultrastructure of fan shell and pearl oyster byssus†
Soft Matter ( IF 3.4 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8sm00821c Delphine Pasche 1, 2, 3, 4 , Nils Horbelt 1, 2, 3, 4 , Frédéric Marin 5, 6, 7, 8 , Sébastien Motreuil 5, 6, 7, 8 , Elena Macías-Sánchez 1, 2, 3, 4 , Giuseppe Falini 9, 10, 11, 12 , Dong Soo Hwang 13, 14, 15, 16 , Peter Fratzl 1, 2, 3, 4 , Matthew James Harrington 1, 2, 3, 4, 9
Soft Matter ( IF 3.4 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8sm00821c Delphine Pasche 1, 2, 3, 4 , Nils Horbelt 1, 2, 3, 4 , Frédéric Marin 5, 6, 7, 8 , Sébastien Motreuil 5, 6, 7, 8 , Elena Macías-Sánchez 1, 2, 3, 4 , Giuseppe Falini 9, 10, 11, 12 , Dong Soo Hwang 13, 14, 15, 16 , Peter Fratzl 1, 2, 3, 4 , Matthew James Harrington 1, 2, 3, 4, 9
Affiliation
|
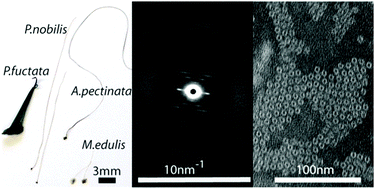
Numerous mussel species produce byssal threads – tough proteinaceous fibers, which anchor mussels in aquatic habitats. Byssal threads from Mytilus species, which are comprised of modified collagen proteins – have become a veritable archetype for bio-inspired polymers due to their self-healing properties. However, threads from different species are comparatively much less understood. In particular, the byssus of Pinna nobilis comprises thousands of fine fibers utilized by humans for millennia to fashion lightweight golden fabrics known as sea silk. P. nobilis is very different from Mytilus from an ecological, morphological and evolutionary point of view and it stands to reason that the structure–function relationships of its byssus are distinct. Here, we performed compositional analysis, X-ray diffraction (XRD) and transmission electron microscopy (TEM) to investigate byssal threads of P. nobilis, as well as a closely related bivalve species (Atrina pectinata) and a distantly related one (Pinctada fucata). This comparative investigation revealed that all three threads share a similar molecular superstructure comprised of globular proteins organized helically into nanofibrils, which is completely distinct from the Mytilus thread ultrastructure, and more akin to the supramolecular organization of bacterial pili and F-actin. This unexpected discovery hints at a possible divergence in byssus evolution in Pinnidae mussels, perhaps related to selective pressures in their respective ecological niches.
中文翻译:

海丝的新变化:扇壳和珍珠贝的独特蛋白质超微结构†
贻贝种类繁多,产生的底线是坚韧的蛋白质纤维,可将贻贝锚定在水生生境中。由Mytilus物种构成的绕线,由于具有自我修复特性,已成为生物启发聚合物的真正原型,由修饰的胶原蛋白组成。但是,来自不同种类的线的了解相对较少。特别地,Pinna nobilis的底纹包含成千上万的人类细纤维,这些纤维被人类使用了数千年,以形成被称为海丝的轻质金色织物。P. nobilis与Mytilus有很大的不同从生态学,形态学和进化学的角度来看,这有理由认为其底栖动物的结构与功能之间的关系是截然不同的。在这里,我们进行了成分分析,X射线衍射(XRD)和透射电子显微镜(TEM)来研究野假单胞菌,近缘的双壳类(Atrina pectinata)和远缘近缘的一种(Pinctada fucata))。这项比较研究表明,所有三个线程都具有相似的分子超结构,该分子超结构由螺旋状组织成纳米纤维的球形蛋白质组成,这与Mytilus完全不同线超微结构,更类似于细菌菌毛和F-肌动蛋白的超分子组织。这一出乎意料的发现暗示了Pinnidae贻贝的沼藻进化可能存在差异,这可能与其各自生态位中的选择性压力有关。
更新日期:2018-06-21
中文翻译:

海丝的新变化:扇壳和珍珠贝的独特蛋白质超微结构†
贻贝种类繁多,产生的底线是坚韧的蛋白质纤维,可将贻贝锚定在水生生境中。由Mytilus物种构成的绕线,由于具有自我修复特性,已成为生物启发聚合物的真正原型,由修饰的胶原蛋白组成。但是,来自不同种类的线的了解相对较少。特别地,Pinna nobilis的底纹包含成千上万的人类细纤维,这些纤维被人类使用了数千年,以形成被称为海丝的轻质金色织物。P. nobilis与Mytilus有很大的不同从生态学,形态学和进化学的角度来看,这有理由认为其底栖动物的结构与功能之间的关系是截然不同的。在这里,我们进行了成分分析,X射线衍射(XRD)和透射电子显微镜(TEM)来研究野假单胞菌,近缘的双壳类(Atrina pectinata)和远缘近缘的一种(Pinctada fucata))。这项比较研究表明,所有三个线程都具有相似的分子超结构,该分子超结构由螺旋状组织成纳米纤维的球形蛋白质组成,这与Mytilus完全不同线超微结构,更类似于细菌菌毛和F-肌动蛋白的超分子组织。这一出乎意料的发现暗示了Pinnidae贻贝的沼藻进化可能存在差异,这可能与其各自生态位中的选择性压力有关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号