当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
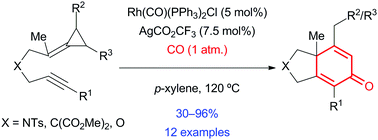
Rhodium-catalyzed [(3+2)+1] carbocyclizations of alkynylidenecyclopropanes with carbon monoxide: construction of polysubstituted bicyclohexa-2,5-dienones†
Chemical Communications ( IF 4.9 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8cc02269k Andrew J. Burnie 1, 2, 3, 4 , P. Andrew Evans 1, 2, 3, 4
Chemical Communications ( IF 4.9 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8cc02269k Andrew J. Burnie 1, 2, 3, 4 , P. Andrew Evans 1, 2, 3, 4
Affiliation
|
A rhodium-catalyzed carbocyclization reaction of alkynylidenecyclopropanes with carbon monoxide to prepare bicyclohexa-2,5-dienones is described. This protocol offers convenient access to doubly conjugated cyclic enones and is tolerant of functionalized alkynes and cyclopropanes. Furthermore, a photochemical rearrangement of a bicyclohexa-2,5-dienone facilitates the construction of a highly functionalized bicyclopentenone containing two contiguous stereogenic centres, which represents a versatile intermediate for target directed synthesis.
中文翻译:

铑用一氧化碳催化炔烃亚环丙烷的[(3 + 2)+1]碳环化:多取代双环己-2,5-二壬烯的构建†
描述了炔属亚烷基环丙烷与一氧化碳的铑催化的碳环化反应以制备双环己-2,5-二壬烯。该协议提供了对双共轭环烯酮的便捷访问,并且可以容忍功能化的炔烃和环丙烷。此外,双环己基2,5-二烯酮的光化学重排促进了包含两个连续的立体异构中心的高度官能化的双环戊烯酮的构建,这代表了用于靶标定向合成的通用中间体。
更新日期:2018-06-21
中文翻译:

铑用一氧化碳催化炔烃亚环丙烷的[(3 + 2)+1]碳环化:多取代双环己-2,5-二壬烯的构建†
描述了炔属亚烷基环丙烷与一氧化碳的铑催化的碳环化反应以制备双环己-2,5-二壬烯。该协议提供了对双共轭环烯酮的便捷访问,并且可以容忍功能化的炔烃和环丙烷。此外,双环己基2,5-二烯酮的光化学重排促进了包含两个连续的立体异构中心的高度官能化的双环戊烯酮的构建,这代表了用于靶标定向合成的通用中间体。


























 京公网安备 11010802027423号
京公网安备 11010802027423号