当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct observation of the kinetics of gas–solid reactions using in situ kinetic and spectroscopic techniques
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2018-06-21 , DOI: 10.1039/c8re00020d Adam S. Hoffman 1, 2, 3, 4 , Sara Azzam 4, 5, 6, 7 , Kai Zhang 1, 2, 3, 4, 8 , Yahong Xu 1, 2, 3, 4 , Yijin Liu 1, 2, 3, 4 , Simon R. Bare 1, 2, 3, 4 , Dante A. Simonetti 4, 5, 6, 7
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2018-06-21 , DOI: 10.1039/c8re00020d Adam S. Hoffman 1, 2, 3, 4 , Sara Azzam 4, 5, 6, 7 , Kai Zhang 1, 2, 3, 4, 8 , Yahong Xu 1, 2, 3, 4 , Yijin Liu 1, 2, 3, 4 , Simon R. Bare 1, 2, 3, 4 , Dante A. Simonetti 4, 5, 6, 7
Affiliation
|
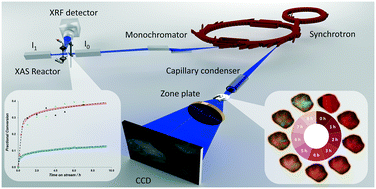
Developing fundamental insight for reactions between gas phase H2S and solid phase CuO has the potential to lead to improved materials and processes for natural gas purification. However, this insight requires detailed knowledge of the atomistic characteristics of the solid and how these characteristics influence the reaction mechanism and kinetics. Herein, we use fixed bed reactors, X-ray absorption spectroscopy, and transmission X-ray microscopy to simultaneously probe the fundamental kinetics of the reaction of CuO with H2S to form CuS, and thereby probe spatial–temporal chemical and structural changes of copper during this reaction. H2S removal reaction kinetics show similar trends in fixed bed reactors as in 10–20 μm sized particles. However, reaction fronts proceed through the entire diameter of particles heterogeneously, indicating the presence of pore diffusion resistance even at very small length scales. In addition, CuO sorbent samples with similar characteristics exhibit 3 times different sulfidation conversion with reaction rate constants that differ by a factor of 1.5. These differences in reaction kinetics and conversion indicate the critical impact of possible atomic scale differences and the formation of different copper sulfide products.
中文翻译:

使用原位动力学和光谱技术直接观察气固反应的动力学
对气相H 2 S和固相CuO之间的反应的发展有了基本的认识,就有可能导致改进天然气净化的材料和工艺。但是,这种见解需要对固体的原子特性以及这些特性如何影响反应机理和动力学的详细了解。在这里,我们使用固定床反应器,X射线吸收光谱和透射X射线显微镜来同时探测CuO与H 2 S形成CuS的反应的基本动力学,从而探测时空化学和结构变化。该反应过程中的铜。高2固定床反应器中的S去除反应动力学显示出与10–20μm大小的颗粒相似的趋势。但是,反应前沿不均匀地穿过颗粒的整个直径,这表明即使在非常小的长度范围内,也存在抗孔扩散性。此外,具有相似特性的CuO吸附剂样品的硫化转化率相差3倍,反应速率常数相差1.5倍。反应动力学和转化率的这些差异表明可能的原子尺度差异和形成不同的硫化铜产物的关键影响。
更新日期:2018-10-03
中文翻译:

使用原位动力学和光谱技术直接观察气固反应的动力学
对气相H 2 S和固相CuO之间的反应的发展有了基本的认识,就有可能导致改进天然气净化的材料和工艺。但是,这种见解需要对固体的原子特性以及这些特性如何影响反应机理和动力学的详细了解。在这里,我们使用固定床反应器,X射线吸收光谱和透射X射线显微镜来同时探测CuO与H 2 S形成CuS的反应的基本动力学,从而探测时空化学和结构变化。该反应过程中的铜。高2固定床反应器中的S去除反应动力学显示出与10–20μm大小的颗粒相似的趋势。但是,反应前沿不均匀地穿过颗粒的整个直径,这表明即使在非常小的长度范围内,也存在抗孔扩散性。此外,具有相似特性的CuO吸附剂样品的硫化转化率相差3倍,反应速率常数相差1.5倍。反应动力学和转化率的这些差异表明可能的原子尺度差异和形成不同的硫化铜产物的关键影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号