当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In situ spectroscopic studies of the one-pot synthesis of composition-controlled Cu–Ni nanowires with enhanced catalytic activity†
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8nj01641k Masanao Ishijima 1, 2, 3, 4 , Jhon L. Cuya Huaman 1, 2, 3, 4 , Shun Yokoyama 4, 5, 6, 7 , Kozo Shinoda 4, 6, 8, 9 , Masahito Uchikoshi 4, 6, 8, 9 , Hiroshi Miyamura 1, 2, 3, 4 , Balachandran Jeyadevan 1, 2, 3, 4
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8nj01641k Masanao Ishijima 1, 2, 3, 4 , Jhon L. Cuya Huaman 1, 2, 3, 4 , Shun Yokoyama 4, 5, 6, 7 , Kozo Shinoda 4, 6, 8, 9 , Masahito Uchikoshi 4, 6, 8, 9 , Hiroshi Miyamura 1, 2, 3, 4 , Balachandran Jeyadevan 1, 2, 3, 4
Affiliation
|
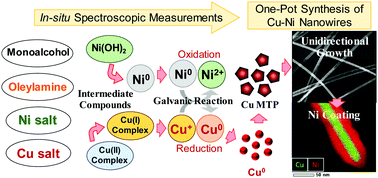
Here, an alcohol reduction method for preparing composition controlled Cu–Ni nanowires (NWs) in high yields is proposed through the selection of an appropriate combination of metallic precursors, alcohol type and surfactant. Also, the mechanism of NW formation was elucidated using morphological, structural and in situ UV-visible and X-ray absorption spectroscopic measurements. In the initial stages of the reaction, the metallic precursors coordinated with amine groups to form complexes. When the temperature of the reactants was increased, Ni formed hydroxide structures such as layered Ni(OH)2, while Cu was partially reduced to Cu+ and coordinated with Cl− ions. At higher temperatures, the Ni2+ ions were reduced to metallic Ni before the formation of metallic Cu. Subsequently, the metallic Ni seeds donated electrons to Cu+ through a galvanic reaction forming Cu seeds. Then, the unidirectional growth progressed due to the etching of the Cu seeds by the chloride ions and the preferential adsorption of the capping agent on the [100] planes of Cu. After the consumption of the Cu ions to form metallic Cu NWs, the Ni ions that remained in the solution were reduced and deposited on the Cu NW surface. The average diameter and length of the Ni-coated Cu NWs were 70 nm and 30 μm, respectively and the composition of Ni varied between 2 and 80 wt%. The catalytic activity of the Cu–Ni NWs was studied through the decoloration reaction of methylene blue, and nanowires with 58 wt% Ni were the most active. These results could be ascribed to the efficient interaction between the Cu core and the Ni sheath.
中文翻译:

原位光谱研究一锅法合成具有增强催化活性的成分控制的Cu-Ni纳米线†
在这里,通过选择适当的金属前体,醇类型和表面活性剂的组合,提出了一种醇还原方法,以高产率制备组成受控的Cu-Ni纳米线(NWs)。此外,使用形态学,结构和原位紫外可见光以及X射线吸收光谱测量来阐明NW形成的机理。在反应的初始阶段,金属前体与胺基配位形成络合物。当反应物的温度升高,镍形成的氢氧化物的结构,例如层状的Ni(OH)2,而Cu进行部分还原为Cu +和有Cl协调-离子。在较高温度下,Ni 2+在形成金属铜之前,将离子还原为金属镍。随后,金属镍种子将电子捐赠给Cu +通过电流反应形成Cu种子。然后,单向生长是由于氯离子对铜晶种的腐蚀以及封端剂在铜的[100]面上的优先吸附而进行的。在消耗铜离子以形成金属铜纳米线之后,残留在溶液中的镍离子被还原并沉积在铜纳米线表面上。镀Ni的铜NW的平均直径和长度分别为70nm和30μm,并且Ni的组成在2至80wt%之间变化。通过亚甲基蓝的脱色反应研究了Cu-Ni NWs的催化活性,其中Ni含量为58%(重量)的纳米线活性最高。这些结果可以归因于铜芯和镍鞘之间的有效相互作用。
更新日期:2018-06-21
中文翻译:

原位光谱研究一锅法合成具有增强催化活性的成分控制的Cu-Ni纳米线†
在这里,通过选择适当的金属前体,醇类型和表面活性剂的组合,提出了一种醇还原方法,以高产率制备组成受控的Cu-Ni纳米线(NWs)。此外,使用形态学,结构和原位紫外可见光以及X射线吸收光谱测量来阐明NW形成的机理。在反应的初始阶段,金属前体与胺基配位形成络合物。当反应物的温度升高,镍形成的氢氧化物的结构,例如层状的Ni(OH)2,而Cu进行部分还原为Cu +和有Cl协调-离子。在较高温度下,Ni 2+在形成金属铜之前,将离子还原为金属镍。随后,金属镍种子将电子捐赠给Cu +通过电流反应形成Cu种子。然后,单向生长是由于氯离子对铜晶种的腐蚀以及封端剂在铜的[100]面上的优先吸附而进行的。在消耗铜离子以形成金属铜纳米线之后,残留在溶液中的镍离子被还原并沉积在铜纳米线表面上。镀Ni的铜NW的平均直径和长度分别为70nm和30μm,并且Ni的组成在2至80wt%之间变化。通过亚甲基蓝的脱色反应研究了Cu-Ni NWs的催化活性,其中Ni含量为58%(重量)的纳米线活性最高。这些结果可以归因于铜芯和镍鞘之间的有效相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号