当前位置:
X-MOL 学术
›
Food Funct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The effect of unadsorbed proteins on the physiochemical properties of the heteroaggregates of oppositely charged lactoferrin coated lutein droplets and whey protein isolate coated DHA droplets
Food & Function ( IF 6.1 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8fo00371h Xin Li 1, 2, 3, 4, 5 , Xu Wang 1, 2, 3, 4, 5 , Jiawei Liu 1, 2, 3, 4, 5 , Duoxia Xu 1, 2, 3, 4, 5 , Yanping Cao 1, 2, 3, 4, 5 , Baoguo Sun 1, 2, 3, 4, 5
Food & Function ( IF 6.1 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8fo00371h Xin Li 1, 2, 3, 4, 5 , Xu Wang 1, 2, 3, 4, 5 , Jiawei Liu 1, 2, 3, 4, 5 , Duoxia Xu 1, 2, 3, 4, 5 , Yanping Cao 1, 2, 3, 4, 5 , Baoguo Sun 1, 2, 3, 4, 5
Affiliation
|
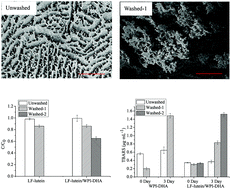
It was reported that the controlled heteroaggregation of oppositely charged lactoferrin (LF)-lutein droplets and whey protein isolate (WPI)-DHA droplets enhanced the physicochemical properties of emulsions. The effect of the unadsorbed proteins on the physicochemical properties of the heteroaggregates of emulsions has not been clear. Therefore, the effects of unadsorbed proteins on the physicochemical stability of heteroaggregates of LF-lutein droplets and WPI-DHA droplets were investigated. The particle size, zeta-potential, transmission-physical stability, microstructure were observed by confocal laser scanning microscopy (CLSM) and cryo-scanning electron microscopy (cryo-SEM) and the chemical stability (lutein degradation and DHA oxidation) of the unwashed and washed heteroaggregates were measured. The results showed that compared with unwashed emulsions, the particle sizes and instability indexes of WPI-DHA emulsions, heteroaggregated LF-lutein/WPI-DHA emulsions and LF-lutein emulsions changed after washing. The instability index of washed-1 heteroaggregated LF-lutein/WPI-DHA emulsion was 10.5 times greater compared with the unwashed samples. The microstructure images showed that the washed single and heteroaggregated emulsions resulted in creaming. The unadsorbed proteins had a great protective effect on the physical stability of the emulsions, especially for the heteroaggregated LF-lutein/WPI-DHA emulsion. The degradation rate of lutein, lipid hydroperoxides and thiobarbituric acid reactive substance (TBARS) values of DHA in washed single and heteroaggregated emulsions were higher than those of the unwashed samples. This proved that the unadsorbed proteins dominated the physicochemical stabilities of heteroaggregates. This laid the foundation for the study of a delivery system of functional component heteroaggregates.
中文翻译:

未吸附蛋白对带相反电荷的乳铁蛋白包被的叶黄素液滴和乳清分离蛋白包被的DHA液滴的杂集的理化性质的影响
据报道,带相反电荷的乳铁蛋白(LF)-叶黄素液滴和乳清蛋白分离物(WPI)-DHA液滴的受控杂聚增强了乳液的理化特性。尚不明确未吸附的蛋白质对乳液杂集料的物理化学性质的影响。因此,研究了未吸附的蛋白质对LF-叶黄素液滴和WPI-DHA液滴的杂集体的理化稳定性的影响。通过共聚焦激光扫描显微镜(CLSM)和冷冻扫描电子显微镜(cryo-SEM)观察了未清洗和未清洗的产品的粒度,ζ电势,透射物理稳定性,微观结构以及化学稳定性(叶黄素降解和DHA氧化)。测量洗涤过的杂聚集体。结果表明,与未洗涤的乳剂相比,洗涤后WPI-DHA乳剂,杂聚集的LF-叶黄素/ WPI-DHA乳剂和LF-叶黄素乳剂的粒径和不稳定性指数发生了变化。与未清洗的样品相比,清洗过的-1杂合的LF-叶黄素/ WPI-DHA乳液的不稳定性指数高10.5倍。显微结构图像显示,洗涤后的单和杂聚集乳液产生了乳脂状。未吸附的蛋白质对乳液的物理稳定性具有很大的保护作用,尤其是对于杂化的LF-叶黄素/ WPI-DHA乳液。叶黄素,脂质过氧化氢和硫代巴比妥酸反应性物质(TBARS)的DHA在洗涤后的单一和杂集乳液中的降解率高于未洗涤样品。这证明了未吸附的蛋白质支配了异质聚集体的物理化学稳定性。这为功能成分杂聚体的递送系统的研究奠定了基础。
更新日期:2018-06-21
中文翻译:

未吸附蛋白对带相反电荷的乳铁蛋白包被的叶黄素液滴和乳清分离蛋白包被的DHA液滴的杂集的理化性质的影响
据报道,带相反电荷的乳铁蛋白(LF)-叶黄素液滴和乳清蛋白分离物(WPI)-DHA液滴的受控杂聚增强了乳液的理化特性。尚不明确未吸附的蛋白质对乳液杂集料的物理化学性质的影响。因此,研究了未吸附的蛋白质对LF-叶黄素液滴和WPI-DHA液滴的杂集体的理化稳定性的影响。通过共聚焦激光扫描显微镜(CLSM)和冷冻扫描电子显微镜(cryo-SEM)观察了未清洗和未清洗的产品的粒度,ζ电势,透射物理稳定性,微观结构以及化学稳定性(叶黄素降解和DHA氧化)。测量洗涤过的杂聚集体。结果表明,与未洗涤的乳剂相比,洗涤后WPI-DHA乳剂,杂聚集的LF-叶黄素/ WPI-DHA乳剂和LF-叶黄素乳剂的粒径和不稳定性指数发生了变化。与未清洗的样品相比,清洗过的-1杂合的LF-叶黄素/ WPI-DHA乳液的不稳定性指数高10.5倍。显微结构图像显示,洗涤后的单和杂聚集乳液产生了乳脂状。未吸附的蛋白质对乳液的物理稳定性具有很大的保护作用,尤其是对于杂化的LF-叶黄素/ WPI-DHA乳液。叶黄素,脂质过氧化氢和硫代巴比妥酸反应性物质(TBARS)的DHA在洗涤后的单一和杂集乳液中的降解率高于未洗涤样品。这证明了未吸附的蛋白质支配了异质聚集体的物理化学稳定性。这为功能成分杂聚体的递送系统的研究奠定了基础。


























 京公网安备 11010802027423号
京公网安备 11010802027423号