当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Antioxidant properties and free radical scavenging mechanisms of cyclocurcumin
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-06-20 00:00:00 , DOI: 10.1039/c8nj01819g Yunkui Li 1, 2, 3, 4, 5 , Marirosa Toscano 5, 6, 7, 8 , Gloria Mazzone 5, 6, 7, 8 , Nino Russo 5, 6, 7, 8
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-06-20 00:00:00 , DOI: 10.1039/c8nj01819g Yunkui Li 1, 2, 3, 4, 5 , Marirosa Toscano 5, 6, 7, 8 , Gloria Mazzone 5, 6, 7, 8 , Nino Russo 5, 6, 7, 8
Affiliation
|
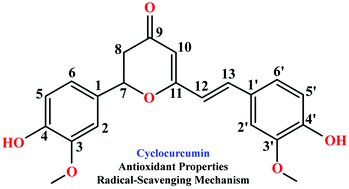
Recently, cyclocurcumin, a seldom studied minor component of curcuminoids, has been found to exhibit abundant bioactivities. To explore the antioxidant properties and free radical scavenging mechanisms of neutral and deprotonated cyclocurcumins, a theoretical study, based on density functional theory using the exchange–correlation M052X functional in connection with the 6-311++G(d,p) basis set, was employed. Their thermodynamic parameters (bond dissociation enthalpy, electron transfer enthalpy, ionization potential, proton affinity and proton dissociation enthalpy) were computed in order to establish what among the hydrogen-atom transfer, single electron transfer-proton transfer and sequential proton loss-electron transfer reaction mechanisms is preferentially followed by cyclocurcumin to inactivate ˙OH and ˙OOH free radicals in water and chlorobenzene. The results show that cyclocurcumin has strong activity as a scavenger of ˙OH and ˙OOH free radicals preferentially by its 4′-OH phenolic radical via a hydrogen-atom transfer mechanism in water and a physiological environment. The solvent effects are not significant for the preferred mechanism.
中文翻译:

环姜黄素的抗氧化性能和清除自由基的机理
最近,很少研究姜黄素的次要成分环姜黄素具有丰富的生物活性。为了探索中性和去质子化的环姜黄素的抗氧化性能和自由基清除机理,基于密度泛函理论的理论研究,使用交换相关M052X官能团与6-311 ++ G(d,p)基组相关联,被雇佣。计算了它们的热力学参数(键解离焓,电子转移焓,电离势,质子亲和力和质子离解焓),以便确定氢原子转移之间的关系,优先进行单电子转移-质子转移和顺序质子损失-电子转移反应机理,然后是环姜黄素使水和氯苯中的OH和OHOH自由基失活。结果表明,环姜黄素具有较强的清除˙OH和˙OOH自由基的活性,优先通过其4'-OH酚基清除剂通过氢原子在水和生理环境中的转移机制。对于优选的机理,溶剂作用并不重要。
更新日期:2018-06-20
中文翻译:

环姜黄素的抗氧化性能和清除自由基的机理
最近,很少研究姜黄素的次要成分环姜黄素具有丰富的生物活性。为了探索中性和去质子化的环姜黄素的抗氧化性能和自由基清除机理,基于密度泛函理论的理论研究,使用交换相关M052X官能团与6-311 ++ G(d,p)基组相关联,被雇佣。计算了它们的热力学参数(键解离焓,电子转移焓,电离势,质子亲和力和质子离解焓),以便确定氢原子转移之间的关系,优先进行单电子转移-质子转移和顺序质子损失-电子转移反应机理,然后是环姜黄素使水和氯苯中的OH和OHOH自由基失活。结果表明,环姜黄素具有较强的清除˙OH和˙OOH自由基的活性,优先通过其4'-OH酚基清除剂通过氢原子在水和生理环境中的转移机制。对于优选的机理,溶剂作用并不重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号