当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sequence‐Based In‐silico Discovery, Characterisation, and Biocatalytic Application of a Set of Imine Reductases
ChemCatChem ( IF 4.5 ) Pub Date : 2018-07-17 , DOI: 10.1002/cctc.201800607 Stefan Velikogne 1 , Verena Resch 1 , Carina Dertnig 1 , Joerg H Schrittwieser 1 , Wolfgang Kroutil 1
ChemCatChem ( IF 4.5 ) Pub Date : 2018-07-17 , DOI: 10.1002/cctc.201800607 Stefan Velikogne 1 , Verena Resch 1 , Carina Dertnig 1 , Joerg H Schrittwieser 1 , Wolfgang Kroutil 1
Affiliation
|
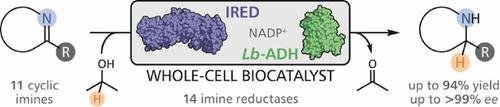
Imine reductases (IREDs) have recently become a primary focus of research in biocatalysis, complementing other classes of amine‐forming enzymes such as transaminases and amine dehydrogenases. Following in the footsteps of other research groups, we have established a set of IRED biocatalysts by sequence‐based in silico enzyme discovery. In this study, we present basic characterisation data for these novel IREDs and explore their activity and stereoselectivity using a panel of structurally diverse cyclic imines as substrates. Specific activities of >1 U/mg and excellent stereoselectivities (ee>99 %) were observed in many cases, and the enzymes proved surprisingly tolerant towards elevated substrate loadings. Co‐expression of the IREDs with an alcohol dehydrogenase for cofactor regeneration led to whole‐cell biocatalysts capable of efficiently reducing imines at 100 mM initial concentration with no need for the addition of extracellular nicotinamide cofactor. Preparative biotransformations on gram scale using these ‘designer cells’ afforded chiral amines in good yield and excellent optical purity.
中文翻译:

一组亚胺还原酶基于序列的计算机发现、表征和生物催化应用
亚胺还原酶(IRED)最近成为生物催化研究的主要焦点,补充了其他类别的胺形成酶,例如转氨酶和胺脱氢酶。跟随其他研究小组的脚步,我们通过基于序列的硅酶发现建立了一套 IRED 生物催化剂。在这项研究中,我们提供了这些新型 IRED 的基本表征数据,并使用一组结构多样的环状亚胺作为底物探索它们的活性和立体选择性。在许多情况下观察到>1 U/mg 的比活性和优异的立体选择性 ( ee >99%),并且这些酶被证明对升高的底物负载具有令人惊讶的耐受性。IRED 与用于辅因子再生的乙醇脱氢酶的共表达导致全细胞生物催化剂能够在 100 mM 初始浓度下有效还原亚胺,而无需添加细胞外烟酰胺辅因子。使用这些“设计细胞”进行克级的制备性生物转化,以良好的产率和优异的光学纯度提供手性胺。
更新日期:2018-07-17
中文翻译:

一组亚胺还原酶基于序列的计算机发现、表征和生物催化应用
亚胺还原酶(IRED)最近成为生物催化研究的主要焦点,补充了其他类别的胺形成酶,例如转氨酶和胺脱氢酶。跟随其他研究小组的脚步,我们通过基于序列的硅酶发现建立了一套 IRED 生物催化剂。在这项研究中,我们提供了这些新型 IRED 的基本表征数据,并使用一组结构多样的环状亚胺作为底物探索它们的活性和立体选择性。在许多情况下观察到>1 U/mg 的比活性和优异的立体选择性 ( ee >99%),并且这些酶被证明对升高的底物负载具有令人惊讶的耐受性。IRED 与用于辅因子再生的乙醇脱氢酶的共表达导致全细胞生物催化剂能够在 100 mM 初始浓度下有效还原亚胺,而无需添加细胞外烟酰胺辅因子。使用这些“设计细胞”进行克级的制备性生物转化,以良好的产率和优异的光学纯度提供手性胺。


























 京公网安备 11010802027423号
京公网安备 11010802027423号