PLOS ONE ( IF 3.7 ) Pub Date : 2018-06-19 , DOI: 10.1371/journal.pone.0199229 John R. Su , Carmen Ng , Paige W. Lewis , Maria V. Cano
|
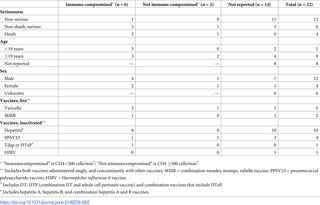
Human immunodeficiency virus (HIV) causes immune dysregulation, potentially affecting response to vaccines in infected persons. We investigated if unexpected adverse events (AEs) or unusual patterns of AEs after vaccination were reported among HIV-positive persons. We searched for domestic reports among HIV-positive persons to the Vaccine Adverse Event Reporting System (VAERS) during 1990–2016. We analyzed reports by age group (<19 and ≥19 years), sex, serious or non-serious status, live vaccine type (live versus inactivated), AEs reported, and CD4 counts. Of 532,235 reports received, 353 (0.07%) described HIV-positive persons, of whom 67% were aged ≥19 years, and 57% were male; most reports (75%) were non-serious. The most commonly reported inactivated vaccines were pneumococcal polysaccharide (27%) and inactivated influenza (27%); the mostly reported common live virus vaccines were combination measles, mumps, and rubella (8%) and varicella (6%). Injection site reactions were commonly reported (39%). Of 67 reports with CD4 counts available, 41 (61%) described persons immunocompromised at time of vaccination (CD4 count <500 cells/mm3), and differed from overall reports only in that varicella was the most common live virus vaccine (4 reports). Of 22 reports describing failure to protect against infection, 6 described persons immunocompromised at time of vaccination, among whom varicella vaccine was most common (3 reports). Of 66 reports describing live virus vaccines, 7 described persons with disseminated infection: 6 had disseminated varicella, 3 of whom had vaccine strain varicella-zoster virus. Of 18 reported deaths, 7 resulted from disseminated infection: 6 were among immunocompromised persons, 1 of whom had vaccine strain varicella-zoster virus. We identified no unexpected or unusual patterns of AEs among HIV-positive persons. These data reinforce current vaccine recommendations for this risk group. However, healthcare providers should know their HIV-positive patients’ immune status because immunocompromising conditions can potentially increase the risk of rare, but severe, AEs following vaccination with live virus vaccines.
中文翻译:

1990-2016年,艾滋病毒呈阳性的人接种疫苗后的不良事件
人类免疫缺陷病毒(HIV)导致免疫功能异常,可能影响感染者对疫苗的反应。我们调查了在HIV阳性者中是否报告了接种疫苗后出现意外不良事件(AE)或异常模式的AE。我们搜索了1990-2016年间在HIV阳性者中疫苗不良事件报告系统(VAERS)的国内报告。我们按年龄组(<19岁和≥19岁),性别,严重或非严重状态,活疫苗类型(活疫苗与灭活疫苗),报道的不良事件和CD4计数分析了报告。在收到的532,235份报告中,有353(0.07%)个描述了HIV阳性的人,其中67%的年龄≥19岁,而57%是男性;大多数报告(75%)是不严重的。最常见的灭活疫苗是肺炎球菌多糖(27%)和灭活流感(27%)。报道最多的常见活病毒疫苗是麻疹,腮腺炎,风疹(8%)和水痘(6%)组合疫苗。注射部位反应通常被报道(39%)。在67个CD4计数报告中,有41个(61%)描述了在接种疫苗时免疫功能低下的人(CD4计数<500细胞/ mm3),与总体报告的不同之处仅在于水痘是最常见的活病毒疫苗(4个报告)。在22篇描述不能预防感染的报告中,有6篇描述了在接种疫苗时免疫功能低下的人,其中以水痘疫苗最为常见(3篇报告)。在描述活病毒疫苗的66份报告中,有7例描述了传播感染的人:6例传播了水痘,其中3例接种了水痘带状疱疹疫苗。在报告的18例死亡中,有7例是由分散感染引起的:免疫功能低下者中有6例,其中1人患有水痘带状疱疹疫苗。我们没有发现艾滋病毒抗体阳性者出现意想不到的或异常的不良事件模式。这些数据加强了针对该风险人群的当前疫苗建议。然而,



























 京公网安备 11010802027423号
京公网安备 11010802027423号