当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unlockable Nanocomplexes with Self‐Accelerating Nucleic Acid Release for Effective Staged Gene Therapy of Cardiovascular Diseases
Advanced Materials ( IF 29.4 ) Pub Date : 2018-06-19 , DOI: 10.1002/adma.201801570 Jing-Jun Nie 1 , Bokang Qiao 2 , Shun Duan 1 , Chen Xu 1 , Boya Chen 2 , Wenjing Hao 2 , Bingran Yu 1 , Yulin Li 2 , Jie Du 2 , Fu-Jian Xu 1
Advanced Materials ( IF 29.4 ) Pub Date : 2018-06-19 , DOI: 10.1002/adma.201801570 Jing-Jun Nie 1 , Bokang Qiao 2 , Shun Duan 1 , Chen Xu 1 , Boya Chen 2 , Wenjing Hao 2 , Bingran Yu 1 , Yulin Li 2 , Jie Du 2 , Fu-Jian Xu 1
Affiliation
|
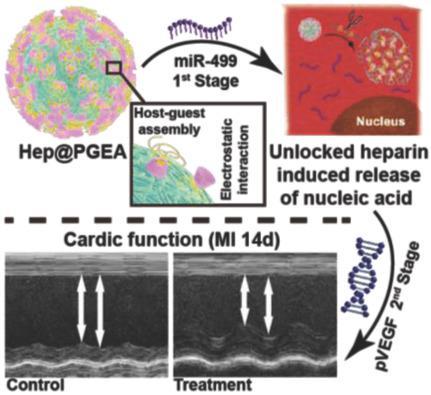
Nucleic acid (NA)‐based therapy is proposed to address serious diseases such as cardiovascular diseases (CVDs). Powerful NA delivery vehicles are essential for effective gene therapy. Herein, a novel type of delivery vehicle, an unlockable core–shell nanocomplex (Hep@PGEA) with self‐accelerating NA release, is structurally designed. Hep@PGEA is composed of disulfide‐bridged heparin nanoparticle (HepNP) core and low‐toxicity PGEA cationic shell. In comparison with NA, heparin, a negatively charged polysaccharide macromolecule, exhibits stronger interactions with cationic species. Upon the breakdown of redox‐responsive HepNP cores, unlocked heparin would interact with the outer cationic shells and replace the condensed NA to facilitate NA release. Such unique Hep@PGEA is successfully explored for effective miRNA–pDNA staged gene therapy of myocardial infarction (MI), one of the most serious CVDs. With the progression of MI, glutathione amounts in heart tissues increase. MiR‐499 (for the inhibition of cardiomyocyte apoptosis) and plasmid encoding vascular endothelial growth factor (for the promotion of angiogenesis) are sequentially delivered for systemic treatment of MI. Such treatment produces impressive results in restoring heart function and suppressing cardiac hypertrophy. Due to the wide existence of redox agents in cells, the proposed unlockable delivery nanovehicle and staged therapy strategy can provide new methods to effectively treat different serious diseases.
中文翻译:

具有自加速核酸释放功能的可解锁纳米复合物,可用于心血管疾病的有效分期基因治疗
建议使用基于核酸(NA)的疗法来解决严重疾病,例如心血管疾病(CVD)。强大的NA传递载体对于有效的基因治疗至关重要。在这里,一种新型的运载工具是一种结构可设计的,具有自加速NA释放功能的可解锁核壳纳米复合物(Hep @ PGEA)。Hep @ PGEA由二硫键桥接的肝素纳米颗粒(HepNP)核心和低毒性PGEA阳离子壳组成。与NA相比,肝素是一种带负电荷的多糖大分子,与阳离子物种的相互作用更强。氧化还原反应性HepNP核心分解后,未锁定的肝素会与阳离子外层相互作用,并取代缩合的NA,以促进NA的释放。这种独特的Hep @ PGEA已成功地探索出用于miRNA-pDNA分级的心肌梗塞(MI)的有效基因治疗,MI是最严重的CVD之一。随着MI的发展,心脏组织中的谷胱甘肽含量增加。MiR-499(用于抑制心肌细胞凋亡)和编码血管内皮生长因子的质粒(用于促进血管生成)被依次递送用于MI的全身治疗。这种治疗在恢复心脏功能和抑制心脏肥大方面产生了令人印象深刻的结果。由于氧化还原剂在细胞中的广泛存在,因此提出的可解锁的递送纳米载体和分阶段的治疗策略可以提供有效治疗不同严重疾病的新方法。MiR-499(用于抑制心肌细胞凋亡)和编码血管内皮生长因子的质粒(用于促进血管生成)被依次递送用于MI的全身治疗。这种治疗在恢复心脏功能和抑制心脏肥大方面产生了令人印象深刻的结果。由于氧化还原剂在细胞中的广泛存在,因此提出的可解锁的递送纳米载体和分阶段的治疗策略可以提供有效治疗不同严重疾病的新方法。MiR-499(用于抑制心肌细胞凋亡)和编码血管内皮生长因子的质粒(用于促进血管生成)依次用于全身性MI治疗。这种治疗在恢复心脏功能和抑制心脏肥大方面产生了令人印象深刻的结果。由于氧化还原剂在细胞中的广泛存在,因此提出的可解锁的递送纳米载体和分阶段的治疗策略可以提供有效治疗不同严重疾病的新方法。
更新日期:2018-06-19
中文翻译:

具有自加速核酸释放功能的可解锁纳米复合物,可用于心血管疾病的有效分期基因治疗
建议使用基于核酸(NA)的疗法来解决严重疾病,例如心血管疾病(CVD)。强大的NA传递载体对于有效的基因治疗至关重要。在这里,一种新型的运载工具是一种结构可设计的,具有自加速NA释放功能的可解锁核壳纳米复合物(Hep @ PGEA)。Hep @ PGEA由二硫键桥接的肝素纳米颗粒(HepNP)核心和低毒性PGEA阳离子壳组成。与NA相比,肝素是一种带负电荷的多糖大分子,与阳离子物种的相互作用更强。氧化还原反应性HepNP核心分解后,未锁定的肝素会与阳离子外层相互作用,并取代缩合的NA,以促进NA的释放。这种独特的Hep @ PGEA已成功地探索出用于miRNA-pDNA分级的心肌梗塞(MI)的有效基因治疗,MI是最严重的CVD之一。随着MI的发展,心脏组织中的谷胱甘肽含量增加。MiR-499(用于抑制心肌细胞凋亡)和编码血管内皮生长因子的质粒(用于促进血管生成)被依次递送用于MI的全身治疗。这种治疗在恢复心脏功能和抑制心脏肥大方面产生了令人印象深刻的结果。由于氧化还原剂在细胞中的广泛存在,因此提出的可解锁的递送纳米载体和分阶段的治疗策略可以提供有效治疗不同严重疾病的新方法。MiR-499(用于抑制心肌细胞凋亡)和编码血管内皮生长因子的质粒(用于促进血管生成)被依次递送用于MI的全身治疗。这种治疗在恢复心脏功能和抑制心脏肥大方面产生了令人印象深刻的结果。由于氧化还原剂在细胞中的广泛存在,因此提出的可解锁的递送纳米载体和分阶段的治疗策略可以提供有效治疗不同严重疾病的新方法。MiR-499(用于抑制心肌细胞凋亡)和编码血管内皮生长因子的质粒(用于促进血管生成)依次用于全身性MI治疗。这种治疗在恢复心脏功能和抑制心脏肥大方面产生了令人印象深刻的结果。由于氧化还原剂在细胞中的广泛存在,因此提出的可解锁的递送纳米载体和分阶段的治疗策略可以提供有效治疗不同严重疾病的新方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号