当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of (−)‐Halenaquinone
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-06-19 , DOI: 10.1002/anie.201805370 Subir Goswami 1 , Kenichi Harada 1, 2 , Mohamed F. El-Mansy 1, 3 , Rajinikanth Lingampally 1 , Rich G. Carter 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-06-19 , DOI: 10.1002/anie.201805370 Subir Goswami 1 , Kenichi Harada 1, 2 , Mohamed F. El-Mansy 1, 3 , Rajinikanth Lingampally 1 , Rich G. Carter 1
Affiliation
|
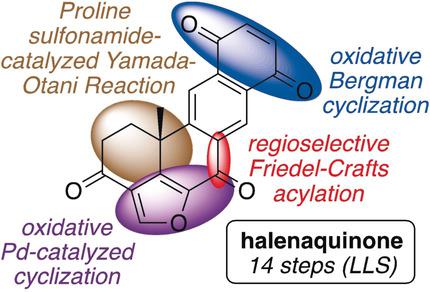
The efficient, 12–14 step (LLS) total synthesis of (−)‐halenaquinone has been achieved. Key steps in the synthetic sequence include: (a) proline sulfonamide‐catalyzed, Yamada–Otani reaction to establish the C6 all‐carbon quaternary stereocenter, (b) multiple, novel palladium‐mediated oxidative cyclizations to introduce the furan moiety, and (c) oxidative Bergman cyclization to form the final quinone ring.
中文翻译:

(-)-哈兰醌的对映选择性合成
已经实现了(-)-卤代苯醌的高效12-14步(LLS)全合成。合成过程中的关键步骤包括:(a)脯氨酸磺酰胺催化的Yamada–Otani反应,以建立C6全碳四元立体中心,(b)多个新颖的钯介导的氧化环化反应,以引入呋喃部分,和(c )氧化伯格曼环化反应形成最终的醌环。
更新日期:2018-06-19
中文翻译:

(-)-哈兰醌的对映选择性合成
已经实现了(-)-卤代苯醌的高效12-14步(LLS)全合成。合成过程中的关键步骤包括:(a)脯氨酸磺酰胺催化的Yamada–Otani反应,以建立C6全碳四元立体中心,(b)多个新颖的钯介导的氧化环化反应,以引入呋喃部分,和(c )氧化伯格曼环化反应形成最终的醌环。



























 京公网安备 11010802027423号
京公网安备 11010802027423号