当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Alanine Scanning of YbdZ, an MbtH-like Protein, Reveals Essential Residues for Functional Interactions with Its Nonribosomal Peptide Synthetase Partner EntF
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1021/acs.biochem.8b00552 Rebecca A. Schomer 1 , Hyunjun Park 1 , John J. Barkei 1 , Michael G. Thomas 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1021/acs.biochem.8b00552 Rebecca A. Schomer 1 , Hyunjun Park 1 , John J. Barkei 1 , Michael G. Thomas 1
Affiliation
|
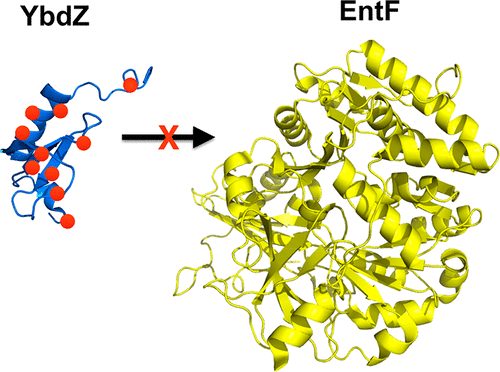
Nonribosomal peptide synthetases (NRPSs) are megasynthetases that require complex and specific interactions between multiple domains and proteins to functionally produce a metabolite. MbtH-like proteins (MLPs) are integral components of many NRPSs and interact directly with the adenylation domain of the megasynthetases to stimulate functional enzymology. All of the MLP residues that are essential for functional interactions between the MLP and NRPS have yet to be defined. Here we probe the interactions between YbdZ, an MLP, and EntF, an NRPS, from Escherichia coli by performing a complete alanine scan of YbdZ. A phenotypic screen identified 11 YbdZ variants that are unable to replace the wild-type MLP, and these YbdZ variants were characterized using a series of in vivo and in vitro assays in an effort to explain why functional interactions with EntF were disrupted. All of the YbdZ variants enhanced the solubility of overproduced EntF, suggesting they were still capable of direct interactions with the megasynthase. Conversely, we show that EntF also influences the solubility of YbdZ and its variants. In vitro biochemical analyses of EntF function with each of the YbdZ variants found the impact that an amino acid substitution will have on NRPS function is difficult to predict, highlighting the complex interaction between these proteins.
中文翻译:

丙氨酸扫描的YbdZ,一种MbtH样蛋白,揭示了其非核糖体肽合成酶伴侣EntF的功能相互作用所必需的残基。
非核糖体肽合成酶(NRPS)是大合成酶,需要多个域和蛋白质之间复杂而特异的相互作用才能功能性地产生代谢产物。MbtH样蛋白(MLP)是许多NRPS不可或缺的组成部分,可直接与巨型合成酶的腺苷酸化域相互作用,以刺激功能性酶学。对于MLP和NRPS之间的功能相互作用必不可少的所有MLP残基都尚未定义。在这里,我们通过对YbdZ进行完全的丙氨酸扫描来探查来自大肠杆菌的YbdZ(一种MLP)和EntF(一种NRPS)之间的相互作用。一个表型筛选确定了11种不能替代野生型MLP的YbdZ变体,并使用一系列体内和体外方法对这些YbdZ变体进行了表征。为了解释为什么与EntF的功能相互作用被破坏的原因,我们进行了体外分析。所有的YbdZ变体都增强了过量生产的EntF的溶解度,表明它们仍然能够与巨合成酶直接相互作用。相反,我们表明EntF也影响YbdZ及其变体的溶解度。每种YbdZ变体对EntF功能的体外生化分析发现,很难预测氨基酸取代对NRPS功能的影响,突出了这些蛋白之间的复杂相互作用。
更新日期:2018-06-19
中文翻译:

丙氨酸扫描的YbdZ,一种MbtH样蛋白,揭示了其非核糖体肽合成酶伴侣EntF的功能相互作用所必需的残基。
非核糖体肽合成酶(NRPS)是大合成酶,需要多个域和蛋白质之间复杂而特异的相互作用才能功能性地产生代谢产物。MbtH样蛋白(MLP)是许多NRPS不可或缺的组成部分,可直接与巨型合成酶的腺苷酸化域相互作用,以刺激功能性酶学。对于MLP和NRPS之间的功能相互作用必不可少的所有MLP残基都尚未定义。在这里,我们通过对YbdZ进行完全的丙氨酸扫描来探查来自大肠杆菌的YbdZ(一种MLP)和EntF(一种NRPS)之间的相互作用。一个表型筛选确定了11种不能替代野生型MLP的YbdZ变体,并使用一系列体内和体外方法对这些YbdZ变体进行了表征。为了解释为什么与EntF的功能相互作用被破坏的原因,我们进行了体外分析。所有的YbdZ变体都增强了过量生产的EntF的溶解度,表明它们仍然能够与巨合成酶直接相互作用。相反,我们表明EntF也影响YbdZ及其变体的溶解度。每种YbdZ变体对EntF功能的体外生化分析发现,很难预测氨基酸取代对NRPS功能的影响,突出了这些蛋白之间的复杂相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号