当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One-pot, highly efficient, cavity controllable synthesis and binding properties of carbazole-based macrocycles with sulfonamide linkages†
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1039/c8qo00538a Deng-Jie Zhu 1, 2, 3, 4 , Wen Ding 1, 2, 3, 4 , Dong-Hui Wang 1, 2, 3, 4 , Min Xue 2, 3, 4, 5 , Yong Yang 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1039/c8qo00538a Deng-Jie Zhu 1, 2, 3, 4 , Wen Ding 1, 2, 3, 4 , Dong-Hui Wang 1, 2, 3, 4 , Min Xue 2, 3, 4, 5 , Yong Yang 1, 2, 3, 4
Affiliation
|
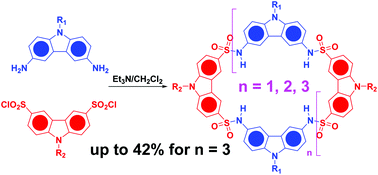
A new type of carbazole-based macrocycle with different dimensions (up to eight carbazole units) was synthesized via formation of sulfonamide linkages. A high cyclization efficiency was achieved under normal synthetic conditions. By introduction of different periphery tails on the carbazole precursors, the product distribution could be substantially adjusted. Moreover, products with specific dimensions could be selectively obtained through a fragment coupling strategy. All the macrocycles showed strong complexation towards the 2,5-dimethoxyterephthalate guest with a 1 : 1 stoichiometry. The binding constants were determined to be of the order of magnitude of 104via UV-vis and FL titrations.
中文翻译:

具有磺酰胺键的咔唑基大环化合物的一锅,高效,腔可控的合成和结合性能†
通过形成磺酰胺键,合成了一种具有不同尺寸(最多八个咔唑单元)的新型咔唑类大环化合物。在正常的合成条件下实现了很高的环化效率。通过在咔唑前体上引入不同的外围尾巴,可以基本上调节产物分布。此外,可以通过片段偶联策略选择性地获得具有特定尺寸的产品。所有的大环化合物都以1:1的化学计量比向2,5-二甲氧基对苯二酸酯客体显示出强烈的络合作用。通过UV-vis和FL滴定确定结合常数为10 4的数量级。
更新日期:2018-06-19
中文翻译:

具有磺酰胺键的咔唑基大环化合物的一锅,高效,腔可控的合成和结合性能†
通过形成磺酰胺键,合成了一种具有不同尺寸(最多八个咔唑单元)的新型咔唑类大环化合物。在正常的合成条件下实现了很高的环化效率。通过在咔唑前体上引入不同的外围尾巴,可以基本上调节产物分布。此外,可以通过片段偶联策略选择性地获得具有特定尺寸的产品。所有的大环化合物都以1:1的化学计量比向2,5-二甲氧基对苯二酸酯客体显示出强烈的络合作用。通过UV-vis和FL滴定确定结合常数为10 4的数量级。



























 京公网安备 11010802027423号
京公网安备 11010802027423号