当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Confinement of H2O and EtOH to enhance CO2 capture in MIL-53(Al)-TDC†
Dalton Transactions ( IF 4 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1039/c8dt01369a Gerardo A. González-Martínez 1, 2, 3, 4, 5 , Tamara Jurado-Vázquez 1, 2, 3, 4, 5 , Diego Solís-Ibarra 1, 2, 3, 4, 5 , Brenda Vargas 1, 2, 3, 4, 5 , Elí Sánchez-González 1, 2, 3, 4, 5 , Ana Martínez 2, 3, 4, 5 , Rubicelia Vargas 4, 5, 6, 7 , Eduardo González-Zamora 4, 5, 6, 7 , Ilich A. Ibarra 1, 2, 3, 4, 5
Dalton Transactions ( IF 4 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1039/c8dt01369a Gerardo A. González-Martínez 1, 2, 3, 4, 5 , Tamara Jurado-Vázquez 1, 2, 3, 4, 5 , Diego Solís-Ibarra 1, 2, 3, 4, 5 , Brenda Vargas 1, 2, 3, 4, 5 , Elí Sánchez-González 1, 2, 3, 4, 5 , Ana Martínez 2, 3, 4, 5 , Rubicelia Vargas 4, 5, 6, 7 , Eduardo González-Zamora 4, 5, 6, 7 , Ilich A. Ibarra 1, 2, 3, 4, 5
Affiliation
|
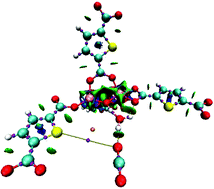
EtOH sorption properties were investigated in MIL-53(Al)-TDC and found a strong interaction between EtOH and the MOF material (ΔHads = 69.6 kJ mol−1). CO2 capture was enhanced upon confining small amounts of H2O. Upon confining small amounts of EtOH however, the CO2 uptake was not improved. The difference in CO2 uptake with EtOH and H2O was rationalised using computational calculations. The analysis of the quantum theory of atoms in molecules (QTAIM) showed a covalent interaction between a MOF model and confined molecules (EtOH and H2O), and no difference in the hydrogen bonds between confined molecules and CO2.
中文翻译:

封闭H 2 O和EtOH以增强MIL-53(Al)-TDC中的CO 2捕获†
在MIL-53(Al)-TDC中研究了EtOH的吸附特性,发现EtOH与MOF材料之间存在很强的相互作用(ΔH ads = 69.6 kJ mol -1)。限制少量H 2 O可以增强CO 2的捕获。然而,限制少量EtOH时,CO 2的吸收并不能提高。使用计算方法可以合理化EtOH和H 2 O吸收CO 2的差异。对分子中原子的量子理论(QTAIM)的分析显示,MOF模型与受限分子(EtOH和H 2 O)之间存在共价相互作用,而受限分子与CO之间的氢键无差异2。
更新日期:2018-06-19
中文翻译:

封闭H 2 O和EtOH以增强MIL-53(Al)-TDC中的CO 2捕获†
在MIL-53(Al)-TDC中研究了EtOH的吸附特性,发现EtOH与MOF材料之间存在很强的相互作用(ΔH ads = 69.6 kJ mol -1)。限制少量H 2 O可以增强CO 2的捕获。然而,限制少量EtOH时,CO 2的吸收并不能提高。使用计算方法可以合理化EtOH和H 2 O吸收CO 2的差异。对分子中原子的量子理论(QTAIM)的分析显示,MOF模型与受限分子(EtOH和H 2 O)之间存在共价相互作用,而受限分子与CO之间的氢键无差异2。



























 京公网安备 11010802027423号
京公网安备 11010802027423号