PLOS ONE ( IF 3.7 ) Pub Date : 2018-06-15 , DOI: 10.1371/journal.pone.0198564 Eyleen Sabine Heidrich , Thomas Brüser
|
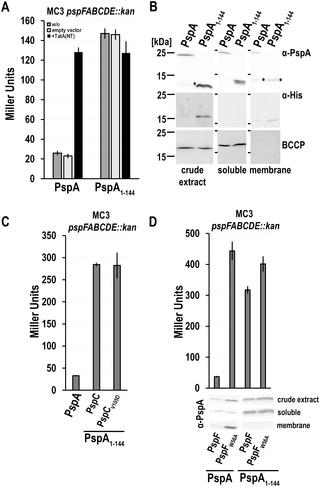
PspA is a key component of the bacterial Psp membrane-stress response system. The biochemical and functional characterization of PspA is impeded by its oligomerization and aggregation properties. It was recently possible to solve the coiled coil structure of a completely soluble PspA fragment, PspA(1–144), that associates with the σ54 enhancer binding protein PspF at its W56-loop and thereby down-regulates the Psp response. We now found that the C-terminal part of PspA, PspA(145–222), also interacts with PspF and inhibits its activity in the absence of full-length PspA. Surprisingly, PspA(145–222) effects changed completely in the presence of full-length PspA, as promoter activity was triggered instead of being inhibited under this condition. PspA(145–222) thus interfered with the inhibitory effect of full-length PspA on PspF, most likely by interacting with full-length PspA that remained bound to PspF. In support of this view, a comprehensive bacterial-2-hybrid screen as well as co-purification analyses indicated a self-interaction of PspA(145–222) and an interaction with full-length PspA. This is the first direct demonstration of PspA/PspA and PspA/PspF interactions in vivo that are mediated by the C-terminus of PspA. The data indicate that regulatory binding sites on PspF do not only exist for the N-terminal coiled coil domain but also for the C-terminal domain of PspA. The inhibition of PspF by PspA-(145–222) was reduced upon membrane stress, whereas the inhibition of PspF by PspA(1–144) did not respond to membrane stress. We therefore propose that the C-terminal domain of PspA is crucial for the regulation of PspF in response to Psp system stimuli.
中文翻译:

PspA的C末端结构域占据的PspF上第二个调控结合位点的证据
PspA是细菌Psp膜应力反应系统的关键组成部分。PspA的低聚和聚集特性阻碍了其的生化和功能表征。这是最近可以解决一个完全可溶PSPA片段的卷曲螺旋结构,PSPA(1-144),其中σ缔合54增强子结合蛋白PspF在其W56环处,从而下调Psp反应。现在我们发现,在没有全长PspA的情况下,PspA的C末端部分PspA(145–222)也可与PspF相互作用并抑制其活性。令人惊讶的是,在存在全长PspA的情况下,PspA(145–222)的作用完全改变,因为启动子活性被触发而不是在此条件下被抑制。因此,PspA(145–222)干扰了全长PspA对PspF的抑制作用,很可能是通过与仍与PspF结合的全长PspA相互作用而产生的。为了支持这种观点,全面的细菌2-杂交筛选以及共纯化分析表明PspA(145–222)的自我相互作用以及与全长PspA的相互作用。这是PspA / PspA和PspA / PspF相互作用的第一个直接演示在体内由PspA的C端介导。数据表明,PspF上的调节结合位点不仅存在于PspA的N末端卷曲螺旋结构域,而且还存在于C末端结构域。PspA-(145–222)对PspF的抑制作用在膜应力作用下有所降低,而PspA(1–144)对PspF的抑制作用对膜应力无反应。因此,我们建议PspA的C末端域对于响应Psp系统刺激而调节PspF至关重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号