当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inductive Effect Boosting Catalytic Performance of Advanced Fe1–xVxOδ Catalysts in Low-Temperature NH3 Selective Catalytic Reduction: Insight into the Structure, Interaction, and Mechanisms
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1021/acscatal.8b01196 Jincheng Mu 1 , Xinyong Li 1 , Wenbo Sun 1 , Shiying Fan 1 , Xinyang Wang 1 , Liang Wang 1 , Meichun Qin 1 , Guoqiang Gan 1 , Zhifan Yin 1 , Dongke Zhang 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1021/acscatal.8b01196 Jincheng Mu 1 , Xinyong Li 1 , Wenbo Sun 1 , Shiying Fan 1 , Xinyang Wang 1 , Liang Wang 1 , Meichun Qin 1 , Guoqiang Gan 1 , Zhifan Yin 1 , Dongke Zhang 2
Affiliation
|
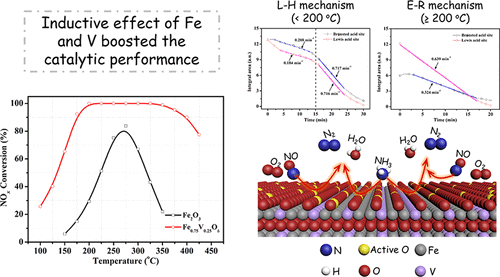
A series of vanadium doped Fe2O3 catalysts were synthesized using the homogeneous precipitation method and subjected to laboratory evaluation for selective catalytic reduction of NOx with NH3 (NH3-SCR). The best Fe0.75V0.25Oδ catalyst with a Fe/V mole ratio of 3/1 exhibited superior catalytic performance, achieving 100% NOx conversion at 200 °C over a wide temperature window from 175 to 400 °C, believed to be the best Fe-based low-temperature NH3-SCR catalyst identified to date. The Fe0.75V0.25Oδ catalyst also showed prominent resistance to high gas hourly space velocity (GHSV; 200 000 h–1) and strong durability to SO2 and H2O. Doping of V was shown to remarkably boost the catalytic activity, due to enhancement of the redox ability and surface acidity. XRD, Raman, and morphology results revealed that the incorporation of V had led to the formation of amorphous FeVO4 and Fe2O3. Coupling XPS and UV–vis diffuse reflectance spectra (DRS) results with DFT, it was discovered that the electron inductive effect between Fe and V generated the charge depletion of Fe, resulting in an improvement of the redox ability, facilitating the oxidation of NO to NO2. Meanwhile, the strong interaction between FeVO4 and Fe2O3 species kept V at a higher valence, beneficial for the adsorption and activation of NH3. The synergistic effect of FeVO4 and Fe2O3 thus improved the low-temperature catalytic activity and lowered the apparent activation energy. Combining in situ diffusion Fourier transform infrared spectroscopy (DRIFTS) results with reaction kinetic studies, it was concluded that the SCR reaction mainly followed the Langmuir–Hinshelwood mechanism below 200 °C, since the consumption of adsorbed NH3 species could be divided into the explicit “standard SCR” and “fast SCR” stages, while an Eley–Rideal mechanism proceeded dominantly at and above 200 °C, in which the adsorbed NH3 species were eliminated by gaseous NO directly and linearly. Both the Brønsted and Lewis acid sites played equivalently significant roles in NH3-SCR reaction.
中文翻译:

诱导效应推进高级的Fe催化性能1个-x V X ø δ催化剂在低温NH 3选择性催化还原:洞察结构,交互和机制
一系列钒掺杂的Fe 2 ö 3个催化剂使用均匀沉淀法合成,并进行实验室评估用于选择性催化还原的NO X与NH 3(NH 3 -SCR)。最好的Fe 0.75 V 0.25 ø δ与3/1由Fe / V摩尔比的催化剂显示出优异的催化性能,实现100%的NO X在200℃下在宽的温度窗口从175℃至400℃转化率,认为是迄今为止确定的最佳铁基低温NH 3 -SCR催化剂。中的Fe 0.75 V 0.25 ö δ催化剂还显示出对高气体时空速度(GHSV; 200 000 h –1)的显着抵抗力,以及对SO 2和H 2 O的强耐久性。由于提高了氧化还原能力,掺杂V可以显着提高催化活性。和表面酸度。XRD,拉曼和形态学结果表明,V的引入导致非晶态的FeVO 4和Fe 2 O 3的形成。将XPS和UV-vis漫反射光谱(DRS)结果与DFT耦合,发现Fe和V之间的电子感应效应产生了Fe的电荷耗尽,从而提高了氧化还原能力,促进了NO氧化为NO 2。同时,FeVO 4和Fe 2 O 3之间的强相互作用使V保持较高的价态,有利于NH 3的吸附和活化。FeVO 4和Fe 2 O 3的协同作用因此改善了低温催化活性并降低了表观活化能。将原位扩散傅里叶变换红外光谱(DRIFTS)结果与反应动力学研究相结合,得出的结论是,SCR反应主要遵循200°C以下的Langmuir-Hinshelwood机理,因为吸收了NH 3物种可分为明确的“标准SCR”和“快速SCR”阶段,而Eley-Rideal机理主要在200°C和更高温度下进行,其中被气态NO直接和线性地消除了吸附的NH 3物种。布朗斯台德和路易斯酸位在NH 3 -SCR反应中均起着相当重要的作用。
更新日期:2018-06-15
中文翻译:

诱导效应推进高级的Fe催化性能1个-x V X ø δ催化剂在低温NH 3选择性催化还原:洞察结构,交互和机制
一系列钒掺杂的Fe 2 ö 3个催化剂使用均匀沉淀法合成,并进行实验室评估用于选择性催化还原的NO X与NH 3(NH 3 -SCR)。最好的Fe 0.75 V 0.25 ø δ与3/1由Fe / V摩尔比的催化剂显示出优异的催化性能,实现100%的NO X在200℃下在宽的温度窗口从175℃至400℃转化率,认为是迄今为止确定的最佳铁基低温NH 3 -SCR催化剂。中的Fe 0.75 V 0.25 ö δ催化剂还显示出对高气体时空速度(GHSV; 200 000 h –1)的显着抵抗力,以及对SO 2和H 2 O的强耐久性。由于提高了氧化还原能力,掺杂V可以显着提高催化活性。和表面酸度。XRD,拉曼和形态学结果表明,V的引入导致非晶态的FeVO 4和Fe 2 O 3的形成。将XPS和UV-vis漫反射光谱(DRS)结果与DFT耦合,发现Fe和V之间的电子感应效应产生了Fe的电荷耗尽,从而提高了氧化还原能力,促进了NO氧化为NO 2。同时,FeVO 4和Fe 2 O 3之间的强相互作用使V保持较高的价态,有利于NH 3的吸附和活化。FeVO 4和Fe 2 O 3的协同作用因此改善了低温催化活性并降低了表观活化能。将原位扩散傅里叶变换红外光谱(DRIFTS)结果与反应动力学研究相结合,得出的结论是,SCR反应主要遵循200°C以下的Langmuir-Hinshelwood机理,因为吸收了NH 3物种可分为明确的“标准SCR”和“快速SCR”阶段,而Eley-Rideal机理主要在200°C和更高温度下进行,其中被气态NO直接和线性地消除了吸附的NH 3物种。布朗斯台德和路易斯酸位在NH 3 -SCR反应中均起着相当重要的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号