当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rigid versus Flexible Protein Matrix: Light-Harvesting Complex II Exhibits a Temperature-Dependent Phonon Spectral Density
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-07-05 , DOI: 10.1021/acs.jpcb.8b02948 Maksym Golub 1 , Leonid Rusevich 2, 3 , Klaus-Dieter Irrgang 4 , Jörg Pieper 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-07-05 , DOI: 10.1021/acs.jpcb.8b02948 Maksym Golub 1 , Leonid Rusevich 2, 3 , Klaus-Dieter Irrgang 4 , Jörg Pieper 1
Affiliation
|
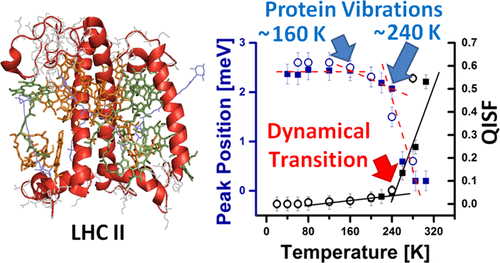
Dynamics-function correlations are usually inferred when molecular mobility and protein function are simultaneously impaired at characteristic temperatures or hydration levels. In this sense, excitation energy transfer in the photosynthetic light-harvesting complex II (LHC II) is an untypical example because it remains fully functional even at cryogenic temperatures relying mainly on interactions of electronic states with protein vibrations. Here, we study the vibrational and conformational protein dynamics of monomeric and trimeric LHC II from spinach using inelastic neutron scattering (INS) in the temperature range of 20–305 K. INS spectra of trimeric LHC II reveal a distinct vibrational peak at ∼2.4 meV. At temperatures above ∼160 K, however, the inelastic peak shifts toward lower energies, which is attributed to vibrational anharmonicity. A more drastic shift is observed at about 240 K, which is interpreted in terms of a “softening” of the protein matrix along with the dynamical transition. Monomeric LHC II exhibits a higher degree of conformational mobility at physiological temperatures, which can be attributed to a higher number of solvent-exposed side chains at the protein surface. The effects of the changes in protein dynamics on the spectroscopic properties of LHC II are considered in comparative model calculations. The absorption line shapes of a pigment molecule embedded into LHC II are simulated for the cases of (i) a rigid protein matrix, (ii) a protein matrix with temperature-dependent spectral density of protein vibrations, and (iii) temperature-dependent electron–phonon coupling strength. Our findings indicate that vibrational and conformational protein dynamics affect the spectroscopic (absorption) properties of LHC II at physiological temperatures.
中文翻译:

刚性与柔性蛋白质基质:捕光复合体II表现出随温度变化的声子光谱密度。
当在特征温度或水合水平同时破坏分子迁移率和蛋白质功能时,通常可以推断出动力学与功能的相关性。从这个意义上讲,光合光捕获复合体II(LHC II)中的激发能转移是一个不典型的例子,因为即使在主要依赖于电子态与蛋白质振动相互作用的低温下,激发能仍能完全发挥作用。在这里,我们在20–305 K的温度范围内使用非弹性中子散射(INS)研究了菠菜中单体和三聚体LHC II的振动和构象蛋白动力学。三聚体LHC II的INS光谱显示了约2.4 meV的明显振动峰。然而,在高于约160 K的温度下,非弹性峰会向较低的能量移动,这归因于振动的非谐性。在约240 K处观察到更剧烈的变化,这可以通过蛋白质基质的“软化”以及动态过渡来解释。单体LHC II在生理温度下表现出更高的构象迁移率,这可以归因于蛋白质表面上更多的溶剂暴露侧链。在比较模型计算中考虑了蛋白质动力学变化对LHC II光谱性质的影响。对于以下情况,模拟了嵌入LHC II的色素分子的吸收线形状:(i)刚性蛋白质基质,(ii)具有温度依赖性蛋白质振动光谱密度的蛋白质基质,以及(iii)温度依赖性电子的情况–声子耦合强度。
更新日期:2018-07-08
中文翻译:

刚性与柔性蛋白质基质:捕光复合体II表现出随温度变化的声子光谱密度。
当在特征温度或水合水平同时破坏分子迁移率和蛋白质功能时,通常可以推断出动力学与功能的相关性。从这个意义上讲,光合光捕获复合体II(LHC II)中的激发能转移是一个不典型的例子,因为即使在主要依赖于电子态与蛋白质振动相互作用的低温下,激发能仍能完全发挥作用。在这里,我们在20–305 K的温度范围内使用非弹性中子散射(INS)研究了菠菜中单体和三聚体LHC II的振动和构象蛋白动力学。三聚体LHC II的INS光谱显示了约2.4 meV的明显振动峰。然而,在高于约160 K的温度下,非弹性峰会向较低的能量移动,这归因于振动的非谐性。在约240 K处观察到更剧烈的变化,这可以通过蛋白质基质的“软化”以及动态过渡来解释。单体LHC II在生理温度下表现出更高的构象迁移率,这可以归因于蛋白质表面上更多的溶剂暴露侧链。在比较模型计算中考虑了蛋白质动力学变化对LHC II光谱性质的影响。对于以下情况,模拟了嵌入LHC II的色素分子的吸收线形状:(i)刚性蛋白质基质,(ii)具有温度依赖性蛋白质振动光谱密度的蛋白质基质,以及(iii)温度依赖性电子的情况–声子耦合强度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号