当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Visible light-induced aryltrifluoromethylation of hydroxy alkenes via radical trifluoromethylation-triggered aryl and heteroaryl migration†
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1039/c8qo00430g Hao Wang 1, 2, 3, 4, 5 , Qian Xu 1, 2, 3, 4, 5 , Shouyun Yu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1039/c8qo00430g Hao Wang 1, 2, 3, 4, 5 , Qian Xu 1, 2, 3, 4, 5 , Shouyun Yu 1, 2, 3, 4, 5
Affiliation
|
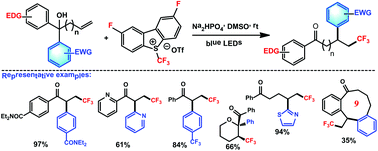
An efficient protocol was developed to achieve the aryltrifluoroalkylation of hydroxy alkenes via radical trifluoromethylation-triggered aryl migration. This method possesses several interesting features: (I) visible light is used as the sole promoter; (II) a new version of Umemoto's reagent [2,8-difluoro-S-(trifluoromethyl)dibenzothiophenium triflate], which is stable, cheap and recyclable, serves as the CF3 source; (III) the aryl migration is chemoselective; (IV) structurally diverse trifluoromethyl ketones can be produced.
中文翻译:

通过自由基三氟甲基化触发的芳基和杂芳基迁移, 可见光诱导的羟基烯烃芳基三氟甲基化†
开发了一种有效的方案,以通过自由基三氟甲基化触发的芳基迁移来实现羟基烯烃的芳基三氟烷基化。该方法具有几个有趣的特征:(I)可见光被用作唯一的促进剂;(II)稳定,廉价且可回收的梅花试剂[2,8-二氟-S-(三氟甲基)二苯并噻吩三氟甲磺酸盐]的新版本可作为CF 3来源;(III)芳基迁移是化学选择性的;(IV)可以生产结构上不同的三氟甲基酮。
更新日期:2018-06-15
中文翻译:

通过自由基三氟甲基化触发的芳基和杂芳基迁移, 可见光诱导的羟基烯烃芳基三氟甲基化†
开发了一种有效的方案,以通过自由基三氟甲基化触发的芳基迁移来实现羟基烯烃的芳基三氟烷基化。该方法具有几个有趣的特征:(I)可见光被用作唯一的促进剂;(II)稳定,廉价且可回收的梅花试剂[2,8-二氟-S-(三氟甲基)二苯并噻吩三氟甲磺酸盐]的新版本可作为CF 3来源;(III)芳基迁移是化学选择性的;(IV)可以生产结构上不同的三氟甲基酮。

























 京公网安备 11010802027423号
京公网安备 11010802027423号