当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
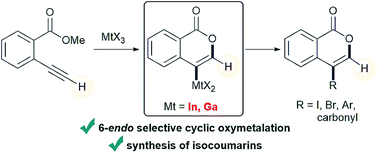
Selective oxymetalation of terminal alkynes via 6-endo cyclization: mechanistic investigation and application to the efficient synthesis of 4-substituted isocoumarins†
Chemical Science ( IF 8.4 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1039/c8sc01537f Yuji Kita 1, 2, 3, 4, 5 , Tetsuji Yata 1, 2, 3, 4, 5 , Yoshihiro Nishimoto 2, 3, 4, 5, 6 , Kouji Chiba 5, 7, 8, 9 , Makoto Yasuda 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1039/c8sc01537f Yuji Kita 1, 2, 3, 4, 5 , Tetsuji Yata 1, 2, 3, 4, 5 , Yoshihiro Nishimoto 2, 3, 4, 5, 6 , Kouji Chiba 5, 7, 8, 9 , Makoto Yasuda 1, 2, 3, 4, 5
Affiliation
|
The cyclization of heteroatom-containing alkynes with π acidic metal salts is an attractive method to prepare heterocycles because the starting materials are readily available and the organometallic compounds are useful synthetic intermediates. A new organometallic species in the heterocyclization provides an opportunity to synthesize heterocycles that are difficult to obtain. Herein, we describe a novel cyclic oxymetalation of 2-alkynylbenzoate with indium or gallium salts that proceeds with an unusual regioselectivity to give isocoumarins bearing a carbon–metal bond at the 4-position. This new type of metalated isocoumarin provided 3-unsubstituted isocoumarins that have seldom been investigated despite their important pharmacological properties. Indium and gallium salts showed high performance in the selective 6-endo cyclization of terminal alkynes while boron or other metals such as Al, Au, and Ag caused 5-exo cyclization or decomposition of terminal alkynes, respectively. The metalated isocoumarin and its reaction intermediate were unambiguously identified by X-ray crystallographic analysis. The theoretical calculation of potential energy profiles showed that oxyindation could proceed via 6-endo cyclization under thermodynamic control while previously reported oxyboration would give a 5-membered ring under kinetic control. The investigation of electrostatic potential maps suggested that the differences in the atomic characters of indium, boron and their ligands would contribute to such a regioselective switch. The metalated isocoumarins were applied to organic synthetic reactions. The halogenation of metalated isocoumarins proceeded to afford 4-halogenated isocoumarins bearing various functional groups. The palladium-catalyzed cross coupling of organometallic species with organic halides gave various 4-substituted isocoumarins. A formal total synthesis of oosponol, which exhibits strong antifungal activity, was accomplished.
中文翻译:

末端炔烃的选择性oxymetalation经由6-内型环化:机械研究和应用到的4-取代的异香豆素的有效合成†
用π酸性金属盐将含杂原子的炔烃环化是制备杂环的一种有吸引力的方法,因为起始原料容易获得并且有机金属化合物是有用的合成中间体。杂环化中的一种新的有机金属物质为合成难以获得的杂环提供了机会。在本文中,我们描述了2-炔基苯甲酸与铟或镓盐的新型环状氧金属化反应,该反应以异常的区域选择性进行,从而得到在4位带有碳-金属键的异香豆素。这种新型的金属化异香豆素提供了3-未取代的异香豆素,尽管它们具有重要的药理特性,但很少进行研究。铟和镓盐在选择性6-内酯中显示出高性能末端炔烃的环化,而硼或其他金属(例如Al,Au和Ag)分别引起末端炔烃的5 exo环化或分解。通过X射线晶体学分析明确鉴定了金属化的香豆素及其反应中间体。势能曲线的理论计算表明,氧代化可以通过6-内酰胺进行在热力学控制下环化,而先前报道的氧硼化将在动力学控制下产生5元环。静电势图的研究表明,铟,硼及其配体的原子特性差异将有助于这种区域选择性开关。金属化的香豆素被用于有机合成反应。进行金属化异香豆素的卤化,得到带有各种官能团的4-卤代异香豆素。钯催化的有机金属物质与有机卤化物的交叉偶联产生了各种4-取代的异香豆素。完成了具有强大抗真菌活性的oosponol的正式全合成。
更新日期:2018-06-15
中文翻译:

末端炔烃的选择性oxymetalation经由6-内型环化:机械研究和应用到的4-取代的异香豆素的有效合成†
用π酸性金属盐将含杂原子的炔烃环化是制备杂环的一种有吸引力的方法,因为起始原料容易获得并且有机金属化合物是有用的合成中间体。杂环化中的一种新的有机金属物质为合成难以获得的杂环提供了机会。在本文中,我们描述了2-炔基苯甲酸与铟或镓盐的新型环状氧金属化反应,该反应以异常的区域选择性进行,从而得到在4位带有碳-金属键的异香豆素。这种新型的金属化异香豆素提供了3-未取代的异香豆素,尽管它们具有重要的药理特性,但很少进行研究。铟和镓盐在选择性6-内酯中显示出高性能末端炔烃的环化,而硼或其他金属(例如Al,Au和Ag)分别引起末端炔烃的5 exo环化或分解。通过X射线晶体学分析明确鉴定了金属化的香豆素及其反应中间体。势能曲线的理论计算表明,氧代化可以通过6-内酰胺进行在热力学控制下环化,而先前报道的氧硼化将在动力学控制下产生5元环。静电势图的研究表明,铟,硼及其配体的原子特性差异将有助于这种区域选择性开关。金属化的香豆素被用于有机合成反应。进行金属化异香豆素的卤化,得到带有各种官能团的4-卤代异香豆素。钯催化的有机金属物质与有机卤化物的交叉偶联产生了各种4-取代的异香豆素。完成了具有强大抗真菌活性的oosponol的正式全合成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号