当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhanced Catalytic Activity of Oxygen‐Tethered IrIII NHC Complexes in Aqueous Transfer Hydrogenative Reductive Amination Reactions: Experimental Kinetic and Mechanistic Study
ChemCatChem ( IF 4.5 ) Pub Date : 2018-07-10 , DOI: 10.1002/cctc.201800558 İbrahim Kayahan Özbozkurt 1 , Derya Gülcemal 1 , Salih Günnaz 1 , Aytaç Gürhan Gökçe 2 , Bekir Çetinkaya 1 , Süleyman Gülcemal 1
ChemCatChem ( IF 4.5 ) Pub Date : 2018-07-10 , DOI: 10.1002/cctc.201800558 İbrahim Kayahan Özbozkurt 1 , Derya Gülcemal 1 , Salih Günnaz 1 , Aytaç Gürhan Gökçe 2 , Bekir Çetinkaya 1 , Süleyman Gülcemal 1
Affiliation
|
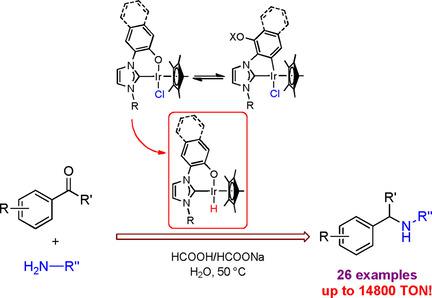
The synthesis and characterization of seven new IrIII complexes containing o‐phenoxide or o‐naphthoxide chelated N‐heterocyclic carbene ligands is reported herein. The crystal structures of six of the complexes have been determined. These complexes efficiently catalyze the transfer hydrogenative reductive amination (RA) of carbonyls and amines in water. Amongst the complexes tested, the introduction of o‐naphthoxide on a nitrogen atom of imidazole based NHC ligand greatly increased the catalytic activity. The catalytic system has a broad substrate scope, which allows the synthesis of a variety of amines in excellent yields and with high turnover numbers up to 490 (for ketones) and 14800 (for aldehydes). The mechanism of aqueous RA reaction with an o‐aryloxide chelated NHC‐IrIII catalyst has been investigated by NMR spectroscopy and kinetic measurements. These studies suggest that the transfer hydrogenation (TH) is turnover‐limited by the hydride formation step. As a result of the 1H NMR studies, the higher catalytic activity of o‐naphthoxide chelated catalyst (3 g) over o‐phenoxide chelated one (3 b) can be attributed partly due to the faster formation of an iridium hydride, the key intermediate in the RA reactions.
中文翻译:

氧束缚的IrIII NHC配合物在水转移加氢还原胺化反应中的催化活性增强:实验动力学和机理研究
本文报道了七种新的Ir III配合物的合成和表征,这些配合物含有邻苯氧或邻萘氧螯合的N杂环卡宾配体。已经确定了六个配合物的晶体结构。这些络合物有效地催化水中羰基和胺的转移氢化还原胺化(RA)。在经过测试的配合物中,引入了o环烷氧基在咪唑基NHC配体的氮原子上大大提高了催化活性。该催化系统具有广泛的底物范围,可以以优异的产率合成多种胺,并具有高达490(对于酮)和14800(对于醛)的高周转率。已经通过NMR光谱和动力学测量研究了RA与邻芳基氧化物螯合的NHC-Ir III催化剂的反应机理。这些研究表明,转移氢化(TH)受氢化物形成步骤的限制。1 H NMR研究的结果表明,邻萘环氧化物螯合催化剂(3 g)的催化活性高于邻苯氧酚被螯合的一(3 b)可以部分归因于氢化铱的快速形成,而氢化铱是RA反应中的关键中间体。
更新日期:2018-07-10
中文翻译:

氧束缚的IrIII NHC配合物在水转移加氢还原胺化反应中的催化活性增强:实验动力学和机理研究
本文报道了七种新的Ir III配合物的合成和表征,这些配合物含有邻苯氧或邻萘氧螯合的N杂环卡宾配体。已经确定了六个配合物的晶体结构。这些络合物有效地催化水中羰基和胺的转移氢化还原胺化(RA)。在经过测试的配合物中,引入了o环烷氧基在咪唑基NHC配体的氮原子上大大提高了催化活性。该催化系统具有广泛的底物范围,可以以优异的产率合成多种胺,并具有高达490(对于酮)和14800(对于醛)的高周转率。已经通过NMR光谱和动力学测量研究了RA与邻芳基氧化物螯合的NHC-Ir III催化剂的反应机理。这些研究表明,转移氢化(TH)受氢化物形成步骤的限制。1 H NMR研究的结果表明,邻萘环氧化物螯合催化剂(3 g)的催化活性高于邻苯氧酚被螯合的一(3 b)可以部分归因于氢化铱的快速形成,而氢化铱是RA反应中的关键中间体。


























 京公网安备 11010802027423号
京公网安备 11010802027423号