Nano Energy ( IF 17.6 ) Pub Date : 2018-06-15 , DOI: 10.1016/j.nanoen.2018.06.039 Chunning Zhao , Meng Yu , Zhi Yang , Jieyu Liu , Shaohua Chen , Zhanglian Hong , Haijun Chen , Weichao Wang
|
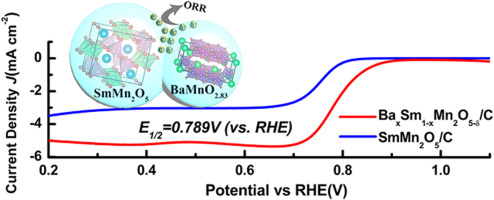
Interface engineering is one of the key strategies to modify the intrinsic electronic structures of catalysts and consequently improve the electrochemical activity of the oxygen reduction reaction (ORR) in the cathodes of the energy conversion devices such as fuel cells and metal-air batteries. Herein, we proposed the mixed-phase mullite (AxSm1−xMn2O5-δ, x = 0–0.5, A=Ca, Sr, Ba), prepared by facile one-step co-precipitation method, to catalyze the oxygen reduction reaction (ORR). The X-ray diffraction (XRD) spectra show that each mixture includes three phases, i.e., mullite SmMn2O5, O-deficient perovskite AMnO3-δ and MnOx. Atomic bonding interfaces are formed between SmMn2O5 and AMnO3-δ, based on the observations of the high resolution transmission electron microscopy (HRTEM). Among these different mixed-phase samples, we find that BaxSm1−xMn2O5-δ/C exhibits the best ORR catalytic activity with the half-wave potential ~ 0.79 V (vs. RHE) and the highest stability over 20,000 s. This performance can be ascribed to the largest charge transfer from BaMnO2.83 to SmMn2O5. Subsequently, partial Mn4+ in mullite SmMn2O5 phase are reduced to active sites Mn3+ to achieve the eg unit occupancy in the interfacial depletion region. In comparison with the reactions over pure-phase mullite SmMn2O5, these transferred electrons are involved into ORR and thus accelerate the proceeding. Our work thus provides insights into designing heterogeneous compound catalysts via interfacing engineering in the applications of the electrochemical oxygen reactions.
中文翻译:

通过与钙钛矿氧化物的界面进行的莫来石SmMn 2 O 5的氧还原反应催化活性的提高
界面工程是修饰催化剂固有电子结构并因此改善诸如燃料电池和金属-空气电池之类的能量转换装置的阴极中的氧还原反应(ORR)的电化学活性的关键策略之一。在此,我们提出了混合相莫来石(A X的Sm 1-x的Mn 2 ö 5-δ中,x = 0-0.5,A =钙,锶,钡),通过容易的一步法共沉淀方法制备的,以催化氧还原反应(ORR)。X射线衍射(XRD)谱表明,每种混合物包括莫来石SmMn 2 O 5,缺氧钙钛矿AMnO3 -δ和MnO x三相。。基于高分辨率透射电子显微镜(HRTEM)的观察结果,SmMn 2 O 5与AMnO3 -δ之间形成了原子键合界面。在这些不同的混合相样品中,我们发现Ba x Sm 1 x Mn 2 O5 -δ / C表现出最佳的ORR催化活性,半波电势约为0.79 V(vs. RHE),在整个过程中具有最高的稳定性。 20,000秒 这种性能可以归因于从BaMnO 2.83到SmMn 2 O 5的最大电荷转移。随后,莫来石SmMn 2 O 5中的部分Mn 4+将相还原成活性位点Mn 3+以达到界面耗尽区的e g单位占有率。与在纯相莫来石SmMn 2 O 5上进行的反应相比,这些转移的电子参与ORR,从而加速了过程。因此,我们的工作为通过电化学氧反应应用中的界面工程设计多相化合物催化剂提供了见识。


























 京公网安备 11010802027423号
京公网安备 11010802027423号