当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modulating Enzyme Activity by Altering Protein Dynamics with Solvent
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-14 00:00:00 , DOI: 10.1021/acs.biochem.8b00424 Michael R. Duff 1 , Jose M. Borreguero 2 , Matthew J. Cuneo 3 , Arvind Ramanathan 4 , Junhong He 5 , Ganesh Kamath 4 , S. Chakra Chennubhotla 6 , Flora Meilleur 3, 7 , Elizabeth E. Howell 1 , Kenneth W. Herwig 5 , Dean A. A. Myles 3 , Pratul K. Agarwal 1, 4
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-14 00:00:00 , DOI: 10.1021/acs.biochem.8b00424 Michael R. Duff 1 , Jose M. Borreguero 2 , Matthew J. Cuneo 3 , Arvind Ramanathan 4 , Junhong He 5 , Ganesh Kamath 4 , S. Chakra Chennubhotla 6 , Flora Meilleur 3, 7 , Elizabeth E. Howell 1 , Kenneth W. Herwig 5 , Dean A. A. Myles 3 , Pratul K. Agarwal 1, 4
Affiliation
|
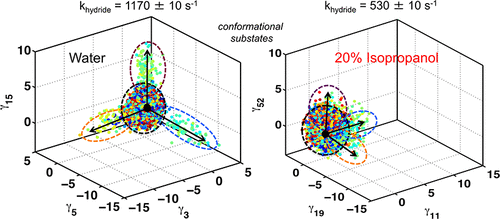
Optimal enzyme activity depends on a number of factors, including structure and dynamics. The role of enzyme structure is well recognized; however, the linkage between protein dynamics and enzyme activity has given rise to a contentious debate. We have developed an approach that uses an aqueous mixture of organic solvent to control the functionally relevant enzyme dynamics (without changing the structure), which in turn modulates the enzyme activity. Using this approach, we predicted that the hydride transfer reaction catalyzed by the enzyme dihydrofolate reductase (DHFR) from Escherichia coli in aqueous mixtures of isopropanol (IPA) with water will decrease by ∼3 fold at 20% (v/v) IPA concentration. Stopped-flow kinetic measurements find that the pH-independent khydride rate decreases by 2.2 fold. X-ray crystallographic enzyme structures show no noticeable differences, while computational studies indicate that the transition state and electrostatic effects were identical for water and mixed solvent conditions; quasi-elastic neutron scattering studies show that the dynamical enzyme motions are suppressed. Our approach provides a unique avenue to modulating enzyme activity through changes in enzyme dynamics. Further it provides vital insights that show the altered motions of DHFR cause significant changes in the enzymeʼs ability to access its functionally relevant conformational substates, explaining the decreased khydride rate. This approach has important implications for obtaining fundamental insights into the role of rate-limiting dynamics in catalysis and as well as for enzyme engineering.
中文翻译:

通过改变蛋白质动力学与溶剂来调节酶活性
最佳酶活性取决于许多因素,包括结构和动力学。酶结构的作用已广为人知;然而,蛋白质动力学与酶活性之间的联系引起了争论。我们已经开发出一种方法,该方法使用有机溶剂的水性混合物控制功能相关的酶动力学(不改变结构),从而调节酶的活性。我们使用这种方法预测,在20%(v / v)IPA浓度下,来自大肠杆菌的二氢叶酸还原酶(DHFR)催化的氢化物转移反应在异丙醇(IPA)与水的水性混合物中将减少约3倍。停止流动力学测量发现不依赖pH的氢化钾比率降低了2.2倍。X射线晶体学酶结构没有明显差异,而计算研究表明,在水和混合溶剂条件下,过渡态和静电效应是相同的。准弹性中子散射研究表明,动态酶运动受到抑制。我们的方法为通过改变酶动力学来调节酶活性提供了独特的途径。此外,它提供了至关重要的见解,表明DHFR的运动改变导致酶进入其功能相关构象亚状态的能力发生了显着变化,从而解释了氢化钾的降低速度。这种方法对于获得对速率限制动力学在催化以及酶工程中的作用的基本见解具有重要意义。
更新日期:2018-06-14
中文翻译:

通过改变蛋白质动力学与溶剂来调节酶活性
最佳酶活性取决于许多因素,包括结构和动力学。酶结构的作用已广为人知;然而,蛋白质动力学与酶活性之间的联系引起了争论。我们已经开发出一种方法,该方法使用有机溶剂的水性混合物控制功能相关的酶动力学(不改变结构),从而调节酶的活性。我们使用这种方法预测,在20%(v / v)IPA浓度下,来自大肠杆菌的二氢叶酸还原酶(DHFR)催化的氢化物转移反应在异丙醇(IPA)与水的水性混合物中将减少约3倍。停止流动力学测量发现不依赖pH的氢化钾比率降低了2.2倍。X射线晶体学酶结构没有明显差异,而计算研究表明,在水和混合溶剂条件下,过渡态和静电效应是相同的。准弹性中子散射研究表明,动态酶运动受到抑制。我们的方法为通过改变酶动力学来调节酶活性提供了独特的途径。此外,它提供了至关重要的见解,表明DHFR的运动改变导致酶进入其功能相关构象亚状态的能力发生了显着变化,从而解释了氢化钾的降低速度。这种方法对于获得对速率限制动力学在催化以及酶工程中的作用的基本见解具有重要意义。

























 京公网安备 11010802027423号
京公网安备 11010802027423号