当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heterogeneous Impacts of Protein-Stabilizing Osmolytes on Hydrophobic Interaction
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-06-28 , DOI: 10.1021/acs.jpcb.8b04654 Mrinmoy Mukherjee 1 , Jagannath Mondal 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-06-28 , DOI: 10.1021/acs.jpcb.8b04654 Mrinmoy Mukherjee 1 , Jagannath Mondal 1
Affiliation
|
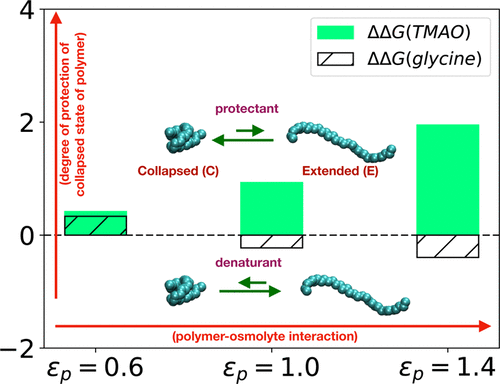
Osmolytes’ mechanism of protecting proteins against denaturation is a longstanding puzzle, further complicated by complex diversities inherent in protein sequences. An emergent approach in understanding the osmolytes’ mechanism of action toward biopolymer has been to investigate osmolytes’ interplay with hydrophobic interaction, the major driving force of protein folding. However, the crucial question is whether all of these protein-stabilizing osmolytes display a single unified mechanism toward hydrophobic interactions. By simulating the hydrophobic collapse of a macromolecule in aqueous solutions of two such osmoprotectants, glycine and trimethyl N-oxide (TMAO), both of which are known to stabilize protein’s folded conformation, we here demonstrate that these two osmolytes can impart mutually contrasting effects toward hydrophobic interaction. Although TMAO preserves its protectant nature across diverse range of polymer–osmolyte interactions, glycine is found to display an interesting crossover from being a protectant at weaker polymer–osmolyte interactions to being a denaturant of hydrophobicity at stronger polymer–osmolyte interactions. A preferential-interaction analysis reveals that a subtle balance of conformation-dependent exclusion/binding of osmolyte molecules from/to the macromolecule holds the key to overall heterogenous behavior. Specifically, TMAOs’ consistent stabilization of collapsed configuration of macromolecule is found to be a result of TMAOs’ preferential binding to polymers via hydrophobic methyl groups. However, polar glycine’s crossover from being a protectant to denaturant across polymer–osmolyte interaction is rooted in its switch from preferential exclusion to preferential binding to the polymer with increasing interaction. Overall, by highlighting the complex interplay of osmolytes with hydrophobic interaction, this work puts forward the necessity of quantitative categorization of osmolytes’ action in protein.
中文翻译:

稳定蛋白质的渗透物对疏水相互作用的异质性影响
Osmolytes保护蛋白质免于变性的机制是一个长期存在的难题,而蛋白质序列固有的复杂多样性使这一问题更加复杂。理解渗透物对生物聚合物的作用机理的一种新兴方法是研究渗透物与疏水相互作用的相互作用,疏水相互作用是蛋白质折叠的主要驱动力。然而,关键的问题是所有这些稳定蛋白质的渗透物是否对疏水性相互作用表现出单一的统一机制。通过模拟两种渗透保护剂甘氨酸和三甲基N的水溶液中大分子的疏水塌陷氧化物(TMAO),两者都已知可以稳定蛋白质的折叠构象,我们在这里证明这两种渗透物可以赋予疏水相互作用互为反差的作用。尽管TMAO在聚合物-渗透物相互作用的各种范围内都保留了其保护性,但发现甘氨酸显示出有趣的跨越,从在较弱的聚合物-渗透物相互作用中作为保护剂转变为在较强的聚合物-渗透物相互作用中为疏水性变性剂。优先相互作用分析表明,构象依赖性的微妙平衡从大分子/向大分子的渗透液分子的排斥/结合是整体异质行为的关键。具体而言,发现TMAOs对大分子塌陷构型的稳定稳定是由于TMAOs通过疏水性甲基优先结合到聚合物上的结果。但是,极性甘氨酸从保护剂到变性剂的跨聚合物-渗透物相互作用的转变,源于其从优先排斥到随着相互作用增加优先与聚合物结合的转变。总的来说,通过强调渗透物与疏水相互作用的复杂相互作用,这项工作提出了对渗透物在蛋白质中作用的定量分类的必要性。
更新日期:2018-06-30
中文翻译:

稳定蛋白质的渗透物对疏水相互作用的异质性影响
Osmolytes保护蛋白质免于变性的机制是一个长期存在的难题,而蛋白质序列固有的复杂多样性使这一问题更加复杂。理解渗透物对生物聚合物的作用机理的一种新兴方法是研究渗透物与疏水相互作用的相互作用,疏水相互作用是蛋白质折叠的主要驱动力。然而,关键的问题是所有这些稳定蛋白质的渗透物是否对疏水性相互作用表现出单一的统一机制。通过模拟两种渗透保护剂甘氨酸和三甲基N的水溶液中大分子的疏水塌陷氧化物(TMAO),两者都已知可以稳定蛋白质的折叠构象,我们在这里证明这两种渗透物可以赋予疏水相互作用互为反差的作用。尽管TMAO在聚合物-渗透物相互作用的各种范围内都保留了其保护性,但发现甘氨酸显示出有趣的跨越,从在较弱的聚合物-渗透物相互作用中作为保护剂转变为在较强的聚合物-渗透物相互作用中为疏水性变性剂。优先相互作用分析表明,构象依赖性的微妙平衡从大分子/向大分子的渗透液分子的排斥/结合是整体异质行为的关键。具体而言,发现TMAOs对大分子塌陷构型的稳定稳定是由于TMAOs通过疏水性甲基优先结合到聚合物上的结果。但是,极性甘氨酸从保护剂到变性剂的跨聚合物-渗透物相互作用的转变,源于其从优先排斥到随着相互作用增加优先与聚合物结合的转变。总的来说,通过强调渗透物与疏水相互作用的复杂相互作用,这项工作提出了对渗透物在蛋白质中作用的定量分类的必要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号