当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The CO2 capturing ability of cellulose dissolved in NaOH(aq) at low temperature
Green Chemistry ( IF 9.8 ) Pub Date : 2018-06-14 , DOI: 10.1039/c8gc01092g Maria Gunnarsson 1, 2, 3, 4, 5 , Diana Bernin 5, 6, 7, 8, 9 , Åsa Östlund 5, 10, 11, 12 , Merima Hasani 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2018-06-14 , DOI: 10.1039/c8gc01092g Maria Gunnarsson 1, 2, 3, 4, 5 , Diana Bernin 5, 6, 7, 8, 9 , Åsa Östlund 5, 10, 11, 12 , Merima Hasani 1, 2, 3, 4, 5
Affiliation
|
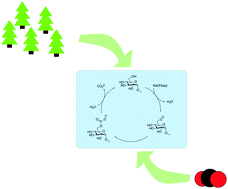
Herein, we explore the intrinsic ability of cellulose dissolved in NaOH(aq) to reversibly capture CO2. The stability of cellulose solutions differed significantly when adding CO2 prior to or after the dissolution of cellulose. ATR-IR spectroscopy on cellulose regenerated from the solutions, using ethanol, revealed the formation of a new carbonate species likely to be cellulose carbonate. To elucidate the interaction of cellulose with CO2 at the molecular level, a 13C NMR spectrum was recorded on methyl α-D-glucopyranoside (MeO-Glcp), a model compound, dissolved in NaOH(aq), which showed a difference in chemical shift when CO2 was added prior to or after the dissolution of MeO-Glcp, without a change in pH. The uptake of CO2 was found to be more than twice as high when CO2 was added to a solution after the dissolution of MeO-Glcp. Altogether, a mechanism for the observed CO2 capture is proposed, involving the formation of an intermediate cellulose carbonate upon the reaction of a cellulose alkoxide with CO2. The intermediate was observed as a captured carbonate structure only in regenerated samples, while its corresponding NMR peak in solution was absent. The reason for this is plausibly a rather fast hydrolysis of the carbonate intermediate by water, leading to the formation of CO32−, and thus increased capture of CO2. The potential of using carbohydrates as CO2 capturing agents in NaOH(aq) is shown to be simple and resource-effective in terms of the capture and regeneration of CO2.
中文翻译:

低温下溶于NaOH(aq)的纤维素的CO 2捕获能力
在这里,我们探讨了溶解在NaOH(aq)中的纤维素可逆地捕获CO 2的内在能力。当在纤维素溶解之前或之后添加CO 2时,纤维素溶液的稳定性显着不同。使用乙醇对从溶液中再生的纤维素进行的ATR-IR光谱分析表明,形成了一种新的碳酸盐物质,可能是碳酸纤维素。为了阐明纤维素与CO 2在分子水平上的相互作用,在溶解于NaOH(aq)的模型化合物甲基α - D-吡喃葡萄糖苷(MeO-Glcp)上记录了13 C NMR光谱。 CO 2时的化学位移在溶解MeO-Glcp之前或之后加入水,pH不变。当在溶解MeO-Glcp后将CO 2添加到溶液中时,发现CO 2的吸收高出两倍以上。总之,提出了用于观察到的CO 2捕获的机制,该机制涉及在纤维素醇盐与CO 2反应时形成中间碳酸纤维素。仅在再生样品中观察到该中间体为捕获的碳酸盐结构,而在溶液中不存在其相应的NMR峰。原因可能是碳酸酯中间体被水相当快地水解,导致形成CO 3 2-,从而增加了对CO的捕集。2。在NaOH(aq)中使用碳水化合物作为CO 2捕获剂的潜力显示出就CO 2的捕获和再生而言既简单又节省资源。
更新日期:2018-07-16
中文翻译:

低温下溶于NaOH(aq)的纤维素的CO 2捕获能力
在这里,我们探讨了溶解在NaOH(aq)中的纤维素可逆地捕获CO 2的内在能力。当在纤维素溶解之前或之后添加CO 2时,纤维素溶液的稳定性显着不同。使用乙醇对从溶液中再生的纤维素进行的ATR-IR光谱分析表明,形成了一种新的碳酸盐物质,可能是碳酸纤维素。为了阐明纤维素与CO 2在分子水平上的相互作用,在溶解于NaOH(aq)的模型化合物甲基α - D-吡喃葡萄糖苷(MeO-Glcp)上记录了13 C NMR光谱。 CO 2时的化学位移在溶解MeO-Glcp之前或之后加入水,pH不变。当在溶解MeO-Glcp后将CO 2添加到溶液中时,发现CO 2的吸收高出两倍以上。总之,提出了用于观察到的CO 2捕获的机制,该机制涉及在纤维素醇盐与CO 2反应时形成中间碳酸纤维素。仅在再生样品中观察到该中间体为捕获的碳酸盐结构,而在溶液中不存在其相应的NMR峰。原因可能是碳酸酯中间体被水相当快地水解,导致形成CO 3 2-,从而增加了对CO的捕集。2。在NaOH(aq)中使用碳水化合物作为CO 2捕获剂的潜力显示出就CO 2的捕获和再生而言既简单又节省资源。



























 京公网安备 11010802027423号
京公网安备 11010802027423号