当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ethoxy‐Ester Functionalized Imidazolium based Ionic Liquids for Lithium Ion Batteries
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-06-14 , DOI: 10.1002/slct.201800513 Trupti Nirmale 1 , Nageshwar Khupse 1 , Rohitkumar Gore 2 , Jalindar Ambekar 1 , Milind Kulkarni 1 , Anjanikumar Varma 3, 4 , Bharat Kale 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-06-14 , DOI: 10.1002/slct.201800513 Trupti Nirmale 1 , Nageshwar Khupse 1 , Rohitkumar Gore 2 , Jalindar Ambekar 1 , Milind Kulkarni 1 , Anjanikumar Varma 3, 4 , Bharat Kale 1
Affiliation
|
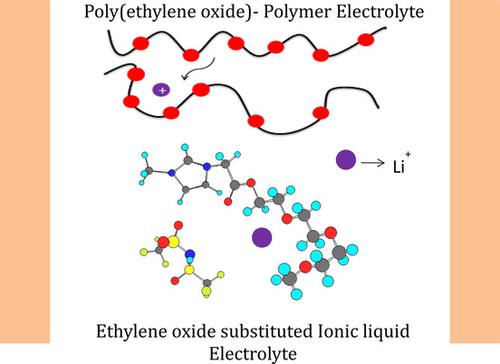
Ethoxy ester functionalized imidazolium and bis(tri fluoromethanesulfonyl)imide based ionic liquids (ILs) are synthesized and considered as electrolyte for lithium ion batteries. The series of ethoxy ester functionalized ionic liquids were chosen with increase in ethoxy unit from one to three, followed by polymeric units. These ionic liquids provide both ester and ethoxy groups as interaction sites for Li+ ions enhancing the Li+ ion transportation, resulting in ionic conductivity of 10−3 Scm−1 at 25 °C, which is of 103 factor higher than ethoxy containing polyethylene oxide solid polymer electrolyte. It's noteworthy that the conductivity increases as ethoxy units are increased from one to three units, followed by a decrease for the polymeric ethoxy unit. Electrochemical stability window of these ionic liquids improves as the ethoxy groups are added to imidazolium cation. The Li/LiFePO4 cell fabricated with [ME3AMIm][TFSI] electrolyte shows good initial discharge capacity of 98.5 mAhg−1 at 0.05 C‐rate at room temperature, which gradually decreases with cycling. Systematic investigation of electrode surfaces by using SEM and EDX shows deposition of passivation layers on their surfaces. Ionic liquids fabricated by this facile method provide a promising model system for understanding the molecular interactions in promoting the lithium‐ion conduction mechanism. The advantages and the limits associated to series of ionic liquid electrolytes are critically investigated.
中文翻译:

锂离子电池的乙氧基酯官能化咪唑鎓离子液体
合成了乙氧基官能化的咪唑鎓和双(三氟甲磺酰基)酰亚胺基离子液体(ILs),并将其视为锂离子电池的电解质。选择乙氧基酯官能化的离子液体系列,其乙氧基单元从一增加到三,然后是聚合物单元。这些离子液体同时提供酯基和乙氧基作为Li +离子的相互作用位点,从而增强Li +离子的运输,在25°C下的离子电导率为10 -3 Scm -1,为10 3系数高于含乙氧基的聚环氧乙烷固体聚合物电解质。值得注意的是,电导率随乙氧基单元从一增加到三个单元而增加,随后聚合乙氧基单元减少。随着乙氧基被添加到咪唑鎓阳离子上,这些离子液体的电化学稳定性窗口得到改善。用[ME 3 AMIm] [TFSI]电解质制备的Li / LiFePO 4电池显示出98.5 mAhg -1的良好初始放电容量在室温下以0.05 C的速率加热,然后随循环逐渐降低。通过使用SEM和EDX对电极表面进行的系统研究表明钝化层在其表面上的沉积。通过这种简便的方法制造的离子液体为理解促进锂离子传导机理的分子相互作用提供了一个有前途的模型系统。严格研究了与一系列离子液体电解质有关的优点和局限性。
更新日期:2018-06-14
中文翻译:

锂离子电池的乙氧基酯官能化咪唑鎓离子液体
合成了乙氧基官能化的咪唑鎓和双(三氟甲磺酰基)酰亚胺基离子液体(ILs),并将其视为锂离子电池的电解质。选择乙氧基酯官能化的离子液体系列,其乙氧基单元从一增加到三,然后是聚合物单元。这些离子液体同时提供酯基和乙氧基作为Li +离子的相互作用位点,从而增强Li +离子的运输,在25°C下的离子电导率为10 -3 Scm -1,为10 3系数高于含乙氧基的聚环氧乙烷固体聚合物电解质。值得注意的是,电导率随乙氧基单元从一增加到三个单元而增加,随后聚合乙氧基单元减少。随着乙氧基被添加到咪唑鎓阳离子上,这些离子液体的电化学稳定性窗口得到改善。用[ME 3 AMIm] [TFSI]电解质制备的Li / LiFePO 4电池显示出98.5 mAhg -1的良好初始放电容量在室温下以0.05 C的速率加热,然后随循环逐渐降低。通过使用SEM和EDX对电极表面进行的系统研究表明钝化层在其表面上的沉积。通过这种简便的方法制造的离子液体为理解促进锂离子传导机理的分子相互作用提供了一个有前途的模型系统。严格研究了与一系列离子液体电解质有关的优点和局限性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号