当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Overcoming Resistance to Targeted Anticancer Therapies through Small-Molecule-Mediated MEK Degradation.
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2018-06-14 , DOI: 10.1016/j.chembiol.2018.05.008 Jessie Peh 1 , Matthew W Boudreau 1 , Hannah M Smith 1 , Paul J Hergenrother 1
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2018-06-14 , DOI: 10.1016/j.chembiol.2018.05.008 Jessie Peh 1 , Matthew W Boudreau 1 , Hannah M Smith 1 , Paul J Hergenrother 1
Affiliation
|
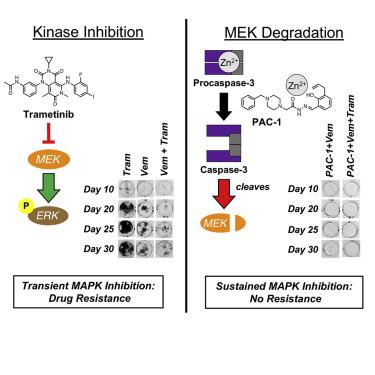
The discovery of mutant or fusion kinases that drive oncogenesis, and the subsequent approval of specific inhibitors for these enzymes, has been instrumental in the management of some cancers. However, acquired resistance remains a significant problem in the clinic, limiting the long-term effectiveness of most of these drugs. Here we demonstrate a general strategy to overcome this resistance through drug-induced MEK cleavage (via direct procaspase-3 activation) combined with targeted kinase inhibition. This combination effect is shown to be general across diverse tumor histologies (melanoma, lung cancer, and leukemia) and driver mutations (mutant BRAF or EGFR, fusion kinases EML4-ALK and BCR-ABL). Caspase-3-mediated degradation of MEK kinases results in sustained pathway inhibition and substantially delayed or eliminated resistance in cancer cells in a manner far superior to combinations with MEK inhibitors. These data suggest the generality of drug-mediated MEK kinase cleavage as a therapeutic strategy to prevent resistance to targeted anticancer therapies.
中文翻译:

通过小分子介导的 MEK 降解克服靶向抗癌治疗的耐药性。
驱动肿瘤发生的突变或融合激酶的发现,以及随后批准的针对这些酶的特定抑制剂,对于某些癌症的治疗发挥了重要作用。然而,获得性耐药仍然是临床上的一个重大问题,限制了大多数这些药物的长期有效性。在这里,我们展示了通过药物诱导的 MEK 裂解(通过直接 procaspase-3 激活)结合靶向激酶抑制来克服这种耐药性的一般策略。这种组合效应在不同的肿瘤组织学(黑色素瘤、肺癌和白血病)和驱动突变(突变 BRAF 或 EGFR、融合激酶 EML4-ALK 和 BCR-ABL)中普遍存在。Caspase-3 介导的 MEK 激酶降解可导致持续的通路抑制,并以远远优于与 MEK 抑制剂组合的方式显着延迟或消除癌细胞的耐药性。这些数据表明药物介导的 MEK 激酶裂解作为预防靶向抗癌疗法耐药性的治疗策略具有普遍性。
更新日期:2018-08-17
中文翻译:

通过小分子介导的 MEK 降解克服靶向抗癌治疗的耐药性。
驱动肿瘤发生的突变或融合激酶的发现,以及随后批准的针对这些酶的特定抑制剂,对于某些癌症的治疗发挥了重要作用。然而,获得性耐药仍然是临床上的一个重大问题,限制了大多数这些药物的长期有效性。在这里,我们展示了通过药物诱导的 MEK 裂解(通过直接 procaspase-3 激活)结合靶向激酶抑制来克服这种耐药性的一般策略。这种组合效应在不同的肿瘤组织学(黑色素瘤、肺癌和白血病)和驱动突变(突变 BRAF 或 EGFR、融合激酶 EML4-ALK 和 BCR-ABL)中普遍存在。Caspase-3 介导的 MEK 激酶降解可导致持续的通路抑制,并以远远优于与 MEK 抑制剂组合的方式显着延迟或消除癌细胞的耐药性。这些数据表明药物介导的 MEK 激酶裂解作为预防靶向抗癌疗法耐药性的治疗策略具有普遍性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号