Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Syntheses of Pyrene-Pyrazole Pharmacophores and Structure Activity Studies for Tubulin Polymerization
ACS Omega ( IF 4.1 ) Pub Date : 2018-06-13 00:00:00 , DOI: 10.1021/acsomega.8b00320 Dinabandhu Sar 1 , Indrajit Srivastava 1 , Santosh K. Misra 1 , Fatemeh Ostadhossein 1 , Parinaz Fathi 1 , Dipanjan Pan 1
ACS Omega ( IF 4.1 ) Pub Date : 2018-06-13 00:00:00 , DOI: 10.1021/acsomega.8b00320 Dinabandhu Sar 1 , Indrajit Srivastava 1 , Santosh K. Misra 1 , Fatemeh Ostadhossein 1 , Parinaz Fathi 1 , Dipanjan Pan 1
Affiliation
|
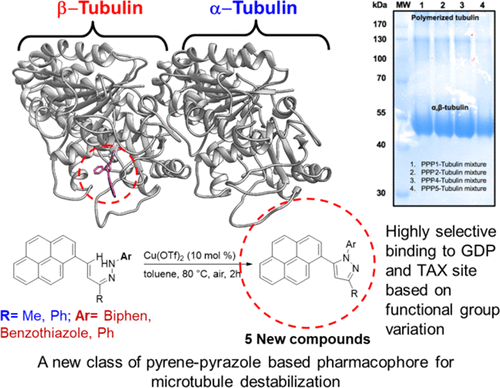
Tubulin polymerization is critical in mitosis process, which regulates uncontrolled cell divisions. Here, we report a new class of pyrene-pyrazole pharmacophore (PPP) for targeting microtubules. Syntheses of seven pyrenyl-substituted pyrazoles with side-chain modification at N-1 and C-3 positions of the pyrazole ring were accomplished from alkenyl hydrazones via C–N dehydrogenative cross-coupling using copper catalyst under aerobic condition. Tubulin polymerization with PPPs was investigated using docking and biological tools to reveal that these ligands are capable of influencing microtubule polymerization and their interaction with α-, β-tubulin active binding sites, which are substituent specific. Furthermore, cytotoxicity response of these PPPs was tested on cancer cells of different origin, such as MCF-7, MDA-MB231, and C32, and also noncancerous normal cells, such as MCF-10A. All newly synthesized PPPs showed excellent anticancer activities. The anticancer activities and half-maximal inhibitory concentration (IC50) values of all PPPs across different cancer cell lines (MCF-7, MDA-MB231, and C32) have been demonstrated. 1,3-Diphenyl-5-(pyren-1-yl)-1H-pyrazole was found to be best among all other PPPs in killing significant population of all of the cancerous cell with IC50 values 1 ± 0.5, 0.5 ± 0.2, and 5.0 ± 2.0 μM in MCF-7, MDA-MB231, and C32 cells, respectively.
中文翻译:

铜催化的-吡唑药基的合成及微管蛋白聚合的结构活性研究
微管蛋白聚合在有丝分裂过程中至关重要,有丝分裂过程调节不受控制的细胞分裂。在这里,我们报告了针对微管的新型class-吡唑药效团(PPP)。在好氧条件下,使用铜催化剂通过C–N脱氢交叉偶联,由烯基生成7个在吡唑环的N-1和C-3位置具有侧链修饰的pyr基取代的吡唑。使用对接和生物学工具研究了具有PPPs的微管蛋白聚合反应,结果表明这些配体能够影响微管聚合及其与取代基特异性的α-,β-微管蛋白活性结合位点的相互作用。此外,在不同来源的癌细胞(例如MCF-7,MDA-MB231和C32)上测试了这些PPP的细胞毒性反应,以及非癌性正常细胞,例如MCF-10A。所有新合成的PPP均显示出优异的抗癌活性。抗癌活性和半数最大抑制浓度(IC已经证明了跨不同癌细胞系(MCF-7,MDA-MB231和C32)的所有PPP的50值。在所有其他PPP中,发现1,3-二苯基-5-(吡喃-1-基)-1 H-吡唑是杀死所有癌细胞的重要种群中最好的,IC 50值为1±0.5、0.5±0.2 ,分别在MCF-7,MDA-MB231和C32细胞中为5.0±2.0μM。
更新日期:2018-06-13
中文翻译:

铜催化的-吡唑药基的合成及微管蛋白聚合的结构活性研究
微管蛋白聚合在有丝分裂过程中至关重要,有丝分裂过程调节不受控制的细胞分裂。在这里,我们报告了针对微管的新型class-吡唑药效团(PPP)。在好氧条件下,使用铜催化剂通过C–N脱氢交叉偶联,由烯基生成7个在吡唑环的N-1和C-3位置具有侧链修饰的pyr基取代的吡唑。使用对接和生物学工具研究了具有PPPs的微管蛋白聚合反应,结果表明这些配体能够影响微管聚合及其与取代基特异性的α-,β-微管蛋白活性结合位点的相互作用。此外,在不同来源的癌细胞(例如MCF-7,MDA-MB231和C32)上测试了这些PPP的细胞毒性反应,以及非癌性正常细胞,例如MCF-10A。所有新合成的PPP均显示出优异的抗癌活性。抗癌活性和半数最大抑制浓度(IC已经证明了跨不同癌细胞系(MCF-7,MDA-MB231和C32)的所有PPP的50值。在所有其他PPP中,发现1,3-二苯基-5-(吡喃-1-基)-1 H-吡唑是杀死所有癌细胞的重要种群中最好的,IC 50值为1±0.5、0.5±0.2 ,分别在MCF-7,MDA-MB231和C32细胞中为5.0±2.0μM。



























 京公网安备 11010802027423号
京公网安备 11010802027423号