当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transformation of lignin model compounds to N-substituted aromatics via Beckmann rearrangement
Green Chemistry ( IF 9.8 ) Pub Date : 2018-06-14 , DOI: 10.1039/c8gc00920a Yinling Wang 1, 2, 3, 4, 5 , Yiman Du 1, 2, 3, 4, 5 , Jianghua He 1, 2, 3, 4, 5 , Yuetao Zhang 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2018-06-14 , DOI: 10.1039/c8gc00920a Yinling Wang 1, 2, 3, 4, 5 , Yiman Du 1, 2, 3, 4, 5 , Jianghua He 1, 2, 3, 4, 5 , Yuetao Zhang 1, 2, 3, 4, 5
Affiliation
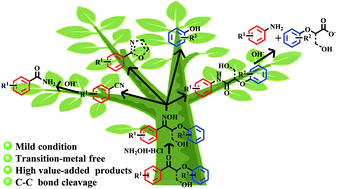
|
Here we present the highly effective cleavage of C–C bonds in lignin model compounds for the production of N-substituted aromatics in up to 96% total yield, including benzonitriles and amides, via oxime formation followed by Beckmann rearrangement (BR). The amides could be further hydrolyzed to anilines (>92% yield) and carboxylic acids (>90% yield), respectively. In addition, the employment of a substrate with a γ-OH group will lead to the formation of C-2 monosubstituted oxazole. A one-pot process involving the BR reaction and hydrolysis has also been developed to directly afford an up to 96% total yield of benzonitriles, benzamides, and anilines. This strategy enabled us to successfully apply the BR reaction to the degradation of lignin model compounds to N-functionalized aromatic products under mild conditions.
中文翻译:

通过贝克曼重排将木质素模型化合物转化为N-取代的芳族化合物
这里,我们提出的C-C键的高度有效的裂解在木质素模型化合物用于生产ñ -取代的芳族化合物在高达96%的总收率,包括苄腈和酰胺,经由肟形成随后贝克曼重排(BR)。酰胺可以分别进一步水解为苯胺(产率> 92%)和羧酸(> 90%)。另外,使用具有γ-OH基团的底物将导致形成C -2单取代的恶唑。还开发了一种涉及BR反应和水解的一锅法,可直接提供高达96%的苯甲腈,苯甲酰胺和苯胺总产率。这种策略使我们能够成功地将BR反应应用于木质素模型化合物的降解在温和条件下经过N-官能化的芳族产品。
更新日期:2018-07-16
中文翻译:

通过贝克曼重排将木质素模型化合物转化为N-取代的芳族化合物
这里,我们提出的C-C键的高度有效的裂解在木质素模型化合物用于生产ñ -取代的芳族化合物在高达96%的总收率,包括苄腈和酰胺,经由肟形成随后贝克曼重排(BR)。酰胺可以分别进一步水解为苯胺(产率> 92%)和羧酸(> 90%)。另外,使用具有γ-OH基团的底物将导致形成C -2单取代的恶唑。还开发了一种涉及BR反应和水解的一锅法,可直接提供高达96%的苯甲腈,苯甲酰胺和苯胺总产率。这种策略使我们能够成功地将BR反应应用于木质素模型化合物的降解在温和条件下经过N-官能化的芳族产品。


























 京公网安备 11010802027423号
京公网安备 11010802027423号