当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
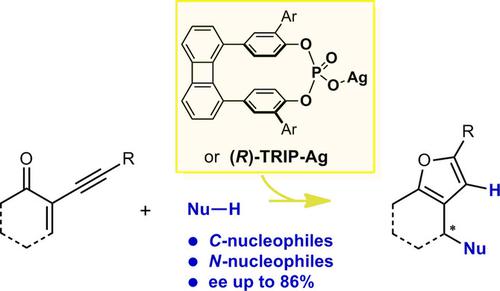
Paracyclophane‐based Silver Phosphates as Catalysts for Enantioselective Cycloisomerization/Addition Reactions: Synthesis of Bicyclic Furans
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-07-11 , DOI: 10.1002/adsc.201800587 Guillaume Force 1 , Yvette Lock Toy Ki 1 , Kévin Isaac 1 , Pascal Retailleau 1 , Angela Marinetti 1 , Jean-François Betzer 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-07-11 , DOI: 10.1002/adsc.201800587 Guillaume Force 1 , Yvette Lock Toy Ki 1 , Kévin Isaac 1 , Pascal Retailleau 1 , Angela Marinetti 1 , Jean-François Betzer 1
Affiliation
|
This manuscript discloses the first use of chiral phosphates based on C2‐symmetric paracyclophane scaffolds as chiral counterions in transition metal catalysis, showing that they may compare favorably with other known chiral phosphates, such as the TRIP phosphate. The targeted catalytic reaction is a silver(I) promoted domino heterocyclization of 2‐(1‐alkynyl)‐2‐alken‐1‐one derivatives, in the presence of C‐, or N‐nucleophiles, which provides an efficient access to substituted bicyclic furans. Results show that high levels of enantioselectivity can be attained with either paracyclophane‐based phosphates or TRIP phosphates, when the nucleophilic reactants display N‐H functions in appropriate positions, near to the nucleophilic center. Therefore, the involvement of H‐bonding between the NH function and the phosphate in the enantiodetermining step is postulated.
中文翻译:

基于对位环烷的磷酸银作为对映选择性环化/加成反应的催化剂:双环呋喃的合成
该手稿公开了基于C2对称对环环烷骨架的手性磷酸盐在过渡金属催化中作为手性抗衡离子的首次使用,表明它们可以与其他已知的手性磷酸盐(如TRIP磷酸盐)相比。目标催化反应是在C-或N存在下,银(I)促进2-(1-炔基)-2-链烯-1-酮衍生物的多米诺骨牌杂环化-亲核试剂,可有效获得取代的双环呋喃。结果表明,当亲核反应物在亲核中心附近的适当位置显示N-H功能时,对环烷基磷酸酯或TRIP磷酸酯均可实现高水平的对映选择性。因此,推测在对映体确定步骤中NH功能和磷酸之间的H键参与。
更新日期:2018-07-11
中文翻译:

基于对位环烷的磷酸银作为对映选择性环化/加成反应的催化剂:双环呋喃的合成
该手稿公开了基于C2对称对环环烷骨架的手性磷酸盐在过渡金属催化中作为手性抗衡离子的首次使用,表明它们可以与其他已知的手性磷酸盐(如TRIP磷酸盐)相比。目标催化反应是在C-或N存在下,银(I)促进2-(1-炔基)-2-链烯-1-酮衍生物的多米诺骨牌杂环化-亲核试剂,可有效获得取代的双环呋喃。结果表明,当亲核反应物在亲核中心附近的适当位置显示N-H功能时,对环烷基磷酸酯或TRIP磷酸酯均可实现高水平的对映选择性。因此,推测在对映体确定步骤中NH功能和磷酸之间的H键参与。


























 京公网安备 11010802027423号
京公网安备 11010802027423号