当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pyrophosphorylation via selective phosphoprotein derivatization†
Chemical Science ( IF 8.4 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1039/c8sc01233d Alan M Marmelstein 1, 2 , Jeremy A M Morgan 1 , Martin Penkert 1, 3 , Daniel T Rogerson 4 , Jason W Chin 4 , Eberhard Krause 1 , Dorothea Fiedler 1, 3
Chemical Science ( IF 8.4 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1039/c8sc01233d Alan M Marmelstein 1, 2 , Jeremy A M Morgan 1 , Martin Penkert 1, 3 , Daniel T Rogerson 4 , Jason W Chin 4 , Eberhard Krause 1 , Dorothea Fiedler 1, 3
Affiliation
|
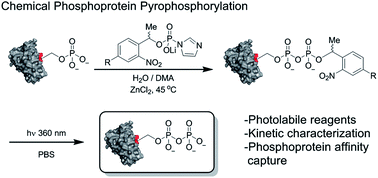
An important step in elucidating the function of protein post-translational modifications (PTMs) is gaining access to site-specifically modified, homogeneous samples for biochemical characterization. Protein pyrophosphorylation is a poorly characterized PTM, and here a chemical approach to obtain pyrophosphoproteins is reported. Photo-labile phosphorimidazolide reagents were developed for selective pyrophosphorylation, affinity-capture, and release of pyrophosphoproteins. Kinetic analysis of the reaction revealed rate constants between 9.2 × 10−3 to 0.58 M−1 s−1, as well as a striking proclivity of the phosphorimidazolides to preferentially react with phosphate monoesters over other nucleophilic side chains. Besides enabling the characterization of pyrophosphorylation on protein function, this work highlights the utility of phosphoryl groups as handles for selective protein modification for a variety of applications, such as phosphoprotein bioconjugation and enrichment.
中文翻译:

通过选择性磷蛋白衍生化进行焦磷酸化†
阐明蛋白质翻译后修饰 (PTM) 功能的一个重要步骤是获得位点特异性修饰的均质样品以进行生化表征。蛋白质焦磷酸化是一种尚不清楚的 PTM,本文报道了一种获得焦磷酸蛋白的化学方法。开发了光不稳定的磷咪唑试剂,用于焦磷酸蛋白的选择性焦磷酸化、亲和捕获和释放。反应动力学分析揭示了9.2 × 10 -3至0.58 M -1 s -1之间的速率常数,以及与其他亲核侧链相比,磷咪唑化物优先与磷酸单酯反应的惊人倾向。除了能够表征焦磷酸化对蛋白质功能的影响外,这项工作还强调了磷酰基作为选择性蛋白质修饰手柄的实用性,适用于各种应用,例如磷蛋白生物共轭和富集。
更新日期:2018-06-12
中文翻译:

通过选择性磷蛋白衍生化进行焦磷酸化†
阐明蛋白质翻译后修饰 (PTM) 功能的一个重要步骤是获得位点特异性修饰的均质样品以进行生化表征。蛋白质焦磷酸化是一种尚不清楚的 PTM,本文报道了一种获得焦磷酸蛋白的化学方法。开发了光不稳定的磷咪唑试剂,用于焦磷酸蛋白的选择性焦磷酸化、亲和捕获和释放。反应动力学分析揭示了9.2 × 10 -3至0.58 M -1 s -1之间的速率常数,以及与其他亲核侧链相比,磷咪唑化物优先与磷酸单酯反应的惊人倾向。除了能够表征焦磷酸化对蛋白质功能的影响外,这项工作还强调了磷酰基作为选择性蛋白质修饰手柄的实用性,适用于各种应用,例如磷蛋白生物共轭和富集。



























 京公网安备 11010802027423号
京公网安备 11010802027423号