当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical Insight into the Kinetics of H‐Abstraction Reaction of CHF2CH2OH with OH Radical, Atmospheric Lifetime and Global Warming Potential
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-06-12 , DOI: 10.1002/slct.201800491 Bidisha Baidya 1 , Makroni Lily 1 , Asit K. Chandra 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-06-12 , DOI: 10.1002/slct.201800491 Bidisha Baidya 1 , Makroni Lily 1 , Asit K. Chandra 1
Affiliation
|
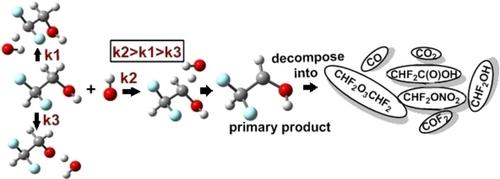
The mechanistic, kinetic and thermochemical analysis of the reaction of CHF2CH2OH with OH radical have been carried out at the CCSD(T)/aug‐cc‐pVTZ//M06‐2X/6‐311++G(d,p) level. The rate coefficients for all the reaction channels are evaluated using conventional transition state theory with Eckart's tunneling correction over a temperature range of 250–500 K. Our calculated rate coefficients agree reasonably well with those reported from the experiments. Heats of formation of CHF2CH2OH molecule and C•F2CH2OH, CHF2C•HOH and CHF2CH2O• radicals have been reported for the first time. Atmospheric lifetime, Global Warming Potential (GWP) and atmospheric degradation pathway of CHF2CH2OH have also been determined in the present study.
中文翻译:

CHF2CH2OH与OH自由基,大气寿命和全球变暖潜能的H-抽象反应动力学的理论研究
CHF 2 CH 2 OH与OH自由基反应的机理,动力学和热化学分析已在CCSD(T)/ aug-cc-pVTZ // M06-2X / 6-311 ++ G(d, p)等级。在250–500 K的温度范围内,使用常规的过渡态理论和Eckart的隧道校正对所有反应通道的速率系数进行评估。我们计算出的速率系数与实验报告的速率系数相当吻合。CHF 2 CH 2 OH分子和C • F 2 CH 2 OH,CHF 2 C • HOH和CHF 2 CH 2 O •的形成热自由基已首次报道。CHF 2 CH 2 OH的大气寿命,全球变暖潜势(GWP)和大气降解途径也已在本研究中确定。
更新日期:2018-06-12
中文翻译:

CHF2CH2OH与OH自由基,大气寿命和全球变暖潜能的H-抽象反应动力学的理论研究
CHF 2 CH 2 OH与OH自由基反应的机理,动力学和热化学分析已在CCSD(T)/ aug-cc-pVTZ // M06-2X / 6-311 ++ G(d, p)等级。在250–500 K的温度范围内,使用常规的过渡态理论和Eckart的隧道校正对所有反应通道的速率系数进行评估。我们计算出的速率系数与实验报告的速率系数相当吻合。CHF 2 CH 2 OH分子和C • F 2 CH 2 OH,CHF 2 C • HOH和CHF 2 CH 2 O •的形成热自由基已首次报道。CHF 2 CH 2 OH的大气寿命,全球变暖潜势(GWP)和大气降解途径也已在本研究中确定。


























 京公网安备 11010802027423号
京公网安备 11010802027423号