Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Clay-Based Nanocomposites as Recyclable Adsorbent toward Hg(II) Capture: Experimental and Theoretical Understanding
ACS Omega ( IF 4.1 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acsomega.8b00789 Sankar Das , Arnab Samanta , Gautam Gangopadhyay , Subhra Jana
ACS Omega ( IF 4.1 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acsomega.8b00789 Sankar Das , Arnab Samanta , Gautam Gangopadhyay , Subhra Jana
|
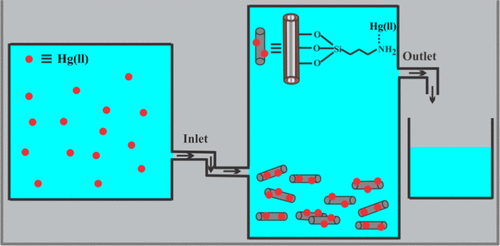
Here, we report the development of inorganic–organic hybrid nanocomposites through selective modification of the negative outer surfaces of halloysite nanoclays with two different organosilanes having primary or secondary amine sites to be explored them as novel and cost-effective adsorbents for the extraction of toxic inorganic contaminants from aqueous solution. They possess excellent selectivity for the adsorption of mercury, which shows monolayer molecular adsorption over the nanocomposites. The adsorption kinetics of Hg(II) is very fast and follows pseudo-second-order model compared to pseudo-first-order model. A combined experimental and theoretical study demonstrated that Hg(II) uptake by these nanocomposites is highly favorable and spontaneous up to 40 °C, and beyond this temperature, the uptake capacity gradually reduced. Temperature-dependent adsorption study exhibits endothermicity at low temperature (≤40 °C) and exothermicity beyond 40 °C. pH-dependent adsorption study showed their high uptake capacity until pH 7, which reduced at alkaline pH. All of the nanocomposites hold excellent adsorption capacity even at low concentration of adsorbate, along with multicycle sorption capability. The outstanding adsorption capacity as well as the easy synthetic route to achieve these nanocomposites may attract researchers to develop low-cost adsorbents to capture toxic metals, which in turn regulate the permissible limit of these toxic metals in drinking water.
中文翻译:

粘土基纳米复合材料作为可回收的汞(II)捕获吸附剂:实验和理论理解
在这里,我们报告了无机-有机杂化纳米复合材料的开发,该方法是通过用两种具有伯胺或仲胺位点的不同有机硅烷对埃洛石纳米粘土的负极外表面进行选择性修饰来进行开发的,将其探索为新颖且经济高效的吸附剂,用于提取有毒无机水溶液中的污染物。它们对汞的吸附具有出色的选择性,显示出在纳米复合材料上的单层分子吸附。Hg(II)的吸附动力学非常快,与拟一阶模型相比,遵循拟二阶模型。结合实验和理论研究表明,这些纳米复合材料对Hg(II)的吸收非常有利,并且在40°C时自发吸收,超过此温度,吸收能力逐渐降低。基于温度的吸附研究显示出在低温(≤40°C)下有吸热性,在超过40°C时有放热性。pH依赖性吸附研究表明,它们的高吸收能力一直到pH 7为止,而在碱性pH值下会降低。所有纳米复合材料即使在低浓度的被吸附物下也具有出色的吸附能力,以及多循环吸附能力。出色的吸附能力以及实现这些纳米复合材料的简便合成途径,可能会吸引研究人员开发低成本的吸附剂来捕获有毒金属,从而反过来调节饮用水中这些有毒金属的允许限量。所有纳米复合材料即使在低浓度的被吸附物下也具有出色的吸附能力,以及多循环吸附能力。出色的吸附能力以及实现这些纳米复合材料的简便合成途径,可能会吸引研究人员开发低成本的吸附剂来捕获有毒金属,从而反过来调节饮用水中这些有毒金属的允许限量。所有纳米复合材料即使在低浓度的被吸附物下也具有出色的吸附能力,以及多循环吸附能力。出色的吸附能力以及实现这些纳米复合材料的简便合成途径,可能会吸引研究人员开发低成本的吸附剂来捕获有毒金属,从而反过来调节饮用水中这些有毒金属的允许限量。
更新日期:2018-06-12
中文翻译:

粘土基纳米复合材料作为可回收的汞(II)捕获吸附剂:实验和理论理解
在这里,我们报告了无机-有机杂化纳米复合材料的开发,该方法是通过用两种具有伯胺或仲胺位点的不同有机硅烷对埃洛石纳米粘土的负极外表面进行选择性修饰来进行开发的,将其探索为新颖且经济高效的吸附剂,用于提取有毒无机水溶液中的污染物。它们对汞的吸附具有出色的选择性,显示出在纳米复合材料上的单层分子吸附。Hg(II)的吸附动力学非常快,与拟一阶模型相比,遵循拟二阶模型。结合实验和理论研究表明,这些纳米复合材料对Hg(II)的吸收非常有利,并且在40°C时自发吸收,超过此温度,吸收能力逐渐降低。基于温度的吸附研究显示出在低温(≤40°C)下有吸热性,在超过40°C时有放热性。pH依赖性吸附研究表明,它们的高吸收能力一直到pH 7为止,而在碱性pH值下会降低。所有纳米复合材料即使在低浓度的被吸附物下也具有出色的吸附能力,以及多循环吸附能力。出色的吸附能力以及实现这些纳米复合材料的简便合成途径,可能会吸引研究人员开发低成本的吸附剂来捕获有毒金属,从而反过来调节饮用水中这些有毒金属的允许限量。所有纳米复合材料即使在低浓度的被吸附物下也具有出色的吸附能力,以及多循环吸附能力。出色的吸附能力以及实现这些纳米复合材料的简便合成途径,可能会吸引研究人员开发低成本的吸附剂来捕获有毒金属,从而反过来调节饮用水中这些有毒金属的允许限量。所有纳米复合材料即使在低浓度的被吸附物下也具有出色的吸附能力,以及多循环吸附能力。出色的吸附能力以及实现这些纳米复合材料的简便合成途径,可能会吸引研究人员开发低成本的吸附剂来捕获有毒金属,从而反过来调节饮用水中这些有毒金属的允许限量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号