当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carbene-catalyzed aerobic oxidation of isoquinolinium salts: efficient synthesis of isoquinolinones
Green Chemistry ( IF 9.8 ) Pub Date : 2018-06-12 , DOI: 10.1039/c8gc01488d Guanjie Wang 1, 2, 3, 4, 5 , Wanyao Hu 1, 2, 3, 4, 5 , Zhouli Hu 1, 2, 3, 4, 5 , Yuxia Zhang 1, 2, 3, 4, 5 , Wei Yao 1, 2, 3, 4, 5 , Lin Li 1, 2, 3, 4, 5 , Zhenqian Fu 1, 2, 3, 4, 5 , Wei Huang 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2018-06-12 , DOI: 10.1039/c8gc01488d Guanjie Wang 1, 2, 3, 4, 5 , Wanyao Hu 1, 2, 3, 4, 5 , Zhouli Hu 1, 2, 3, 4, 5 , Yuxia Zhang 1, 2, 3, 4, 5 , Wei Yao 1, 2, 3, 4, 5 , Lin Li 1, 2, 3, 4, 5 , Zhenqian Fu 1, 2, 3, 4, 5 , Wei Huang 1, 2, 3, 4, 5
Affiliation
|
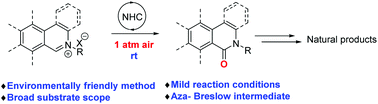
A mild and environmentally friendly carbene-catalyzed aerobic oxidation of isoquinolinium salts was successfully realized. Accordingly, a diverse set of isoquinolinones and phenanthridinones was efficiently prepared in good to excellent yields. The mechanistic study indicates that the formation of an aza-Breslow intermediate is the crucial step in this transformation. This reaction features ambient air as the sole oxidant and oxygen source, a broad substrate scope, and excellent functional-group tolerance and proceeds under mild reaction conditions. Furthermore, a highly efficient synthesis of bioactive molecules and natural products including N-methylcrinasiadine, N-isopentylcrinasiadine, N-phenethylcrinasiadine, isoindolo[2,1-b]isoquinolin-5(7H)-one, PJ-34, rac-Gusanlung D, rosettacin, 8-oxopseudopalmatine and ilicifoline B was accomplished.
中文翻译:

碳催化的异喹啉鎓盐的好氧氧化:异喹啉酮的有效合成
成功实现了温和环保的卡宾催化的异喹啉鎓盐的好氧氧化。因此,有效地制备了各种异喹啉酮和菲啶酮类,收率良好至优异。机理研究表明,氮杂-布雷斯洛中间体的形成是该转变的关键步骤。该反应具有环境空气作为唯一的氧化剂和氧气源,广泛的底物范围以及出色的官能团耐受性的特点,并且可以在温和的反应条件下进行。此外,生物活性分子和天然产物,包括一种高效合成Ñ -methylcrinasiadine,Ñ -isopentylcrinasiadine,Ñ -phenethylcrinasiadine,异吲哚基[2,1- b完成了] isoquinolin -5(7 H)-1,PJ-34,rac-Gusanlung D,罗塞他星,8-氧代伪巴马汀和伊利福林B的合成。
更新日期:2018-07-16
中文翻译:

碳催化的异喹啉鎓盐的好氧氧化:异喹啉酮的有效合成
成功实现了温和环保的卡宾催化的异喹啉鎓盐的好氧氧化。因此,有效地制备了各种异喹啉酮和菲啶酮类,收率良好至优异。机理研究表明,氮杂-布雷斯洛中间体的形成是该转变的关键步骤。该反应具有环境空气作为唯一的氧化剂和氧气源,广泛的底物范围以及出色的官能团耐受性的特点,并且可以在温和的反应条件下进行。此外,生物活性分子和天然产物,包括一种高效合成Ñ -methylcrinasiadine,Ñ -isopentylcrinasiadine,Ñ -phenethylcrinasiadine,异吲哚基[2,1- b完成了] isoquinolin -5(7 H)-1,PJ-34,rac-Gusanlung D,罗塞他星,8-氧代伪巴马汀和伊利福林B的合成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号