Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of Surface Oxygen Vacancies and Lanthanide Contraction Phenomenon of Ln(OH)3 (Ln = La, Pr, and Nd) in Sulfide-Mediated Photoelectrochemical Water Splitting
ACS Omega ( IF 4.1 ) Pub Date : 2018-06-11 00:00:00 , DOI: 10.1021/acsomega.8b00429 Suresh Reddy Sanivarapu 1 , John Berchmans Lawrence 2 , Gosipathala Sreedhar 2
ACS Omega ( IF 4.1 ) Pub Date : 2018-06-11 00:00:00 , DOI: 10.1021/acsomega.8b00429 Suresh Reddy Sanivarapu 1 , John Berchmans Lawrence 2 , Gosipathala Sreedhar 2
Affiliation
|
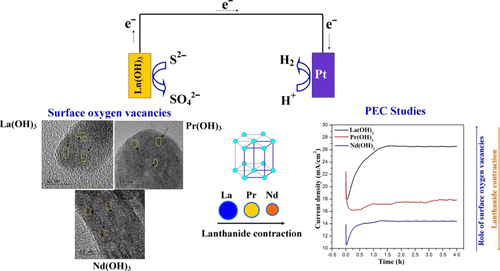
Herein, we report the role of surface oxygen vacancies and lanthanide contraction phenomenon on HS– anion adsorption and desorption in the sulfide-mediated photoelectrochemical water splitting of Ln(OH)3 (Ln = La, Pr, and Nd). The Ln(OH)3 were synthesized via a solvothermal route using ethylenediamine as the solvent. The surface defects are characterized by Raman, X-ray photoelectron spectroscopy (XPS), electron paramagnetic resonance (EPR), and high-resolution transmission electron microscopy analyses. The photoelectrochemical water-splitting behavior of Ln(OH)3 enriched with surface oxygen vacancies has been examined in a 1 M Na2S solution under illumination conditions. La(OH)3 exhibited a highly stable and saturated current density of ∼26 mA/cm2 at 0.8 V (vs Ag/AgCl). Similarly, the hydroxides of Pr and Nd demonstrated current densities of 18 and 14 mA/cm2, respectively, at 0.8 V (vs Ag/AgCl). A reduction trend in the saturated current densities from La to Nd indicates the lanthanide contraction phenomenon, where the basicity decreases in the same order. The results also demonstrate that the surface adsorption of the HS– anion in the active sites of the surface oxygen vacancies played a vital role in enhancing the photoelectrochemical water-splitting behavior of Ln(OH)3. The stability of Ln(OH)3 was examined after 4 h of chronoamperometry studies at 0.8 V (vs Ag/AgCl) and analyzed using X-ray diffraction, Fourier transform infrared, Raman, and EPR and XPS analyses. The results show that the Ln(OH)3 exhibited excellent stability by demonstrating their phase purity after photoelectrochemical water splitting. We propose Ln(OH)3 as highly stable photoelectrochemical water-splitting catalysts in highly concentrated sulfide-based electrolytes and anticipate Ln(OH)3 systems to be explored in a major scale for the production of H2 as an ecofriendly process.
中文翻译:

Ln(OH)3(Ln = La,Pr和Nd)的表面氧空位和镧系元素收缩现象在硫化物介导的光电化学水分解中的作用
在这里,我们报告表面氧空位和镧系元素收缩现象对Ln(OH)3(Ln = La,Pr和Nd)的硫化物介导的光电化学水分解中HS –阴离子吸附和解吸的作用。使用乙二胺作为溶剂,通过溶剂热途径合成了Ln(OH)3。表面缺陷的特征在于拉曼光谱,X射线光电子能谱(XPS),电子顺磁共振(EPR)和高分辨率透射电子显微镜分析。已经在光照条件下的1 M Na 2 S溶液中研究了富含表面氧空位的Ln(OH)3的光电化学水分解行为。镧(OH)3在0.8 V(vs Ag / AgCl)时表现出〜26 mA / cm 2的高度稳定和饱和电流密度。类似地,Pr和Nd的氢氧化物在0.8 V(vs Ag / AgCl)时的电流密度分别为18和14 mA / cm 2。从La到Nd的饱和电流密度的减小趋势表明镧系元素收缩现象,其中碱度以相同的顺序降低。该结果还表明,HS的表面吸附-阴离子表面氧空位的活性位点在提高LN(OH)的光电化学水分解行为发挥了重要作用3。Ln(OH)3的稳定性在0.8 V(vs Ag / AgCl)进行计时电流法研究4小时后,对样品进行了分析,并使用X射线衍射,傅立叶变换红外光谱,拉曼光谱以及EPR和XPS分析进行了分析。结果表明,Ln(OH)3通过证明其在光电化学水分解后的相纯度表现出极好的稳定性。我们提出了Ln(OH)3作为高浓度硫化物基电解质中的高度稳定的光电化学水分解催化剂,并预期Ln(OH)3系统将被大规模探索以生产H 2作为一种生态友好的方法。
更新日期:2018-06-11
中文翻译:

Ln(OH)3(Ln = La,Pr和Nd)的表面氧空位和镧系元素收缩现象在硫化物介导的光电化学水分解中的作用
在这里,我们报告表面氧空位和镧系元素收缩现象对Ln(OH)3(Ln = La,Pr和Nd)的硫化物介导的光电化学水分解中HS –阴离子吸附和解吸的作用。使用乙二胺作为溶剂,通过溶剂热途径合成了Ln(OH)3。表面缺陷的特征在于拉曼光谱,X射线光电子能谱(XPS),电子顺磁共振(EPR)和高分辨率透射电子显微镜分析。已经在光照条件下的1 M Na 2 S溶液中研究了富含表面氧空位的Ln(OH)3的光电化学水分解行为。镧(OH)3在0.8 V(vs Ag / AgCl)时表现出〜26 mA / cm 2的高度稳定和饱和电流密度。类似地,Pr和Nd的氢氧化物在0.8 V(vs Ag / AgCl)时的电流密度分别为18和14 mA / cm 2。从La到Nd的饱和电流密度的减小趋势表明镧系元素收缩现象,其中碱度以相同的顺序降低。该结果还表明,HS的表面吸附-阴离子表面氧空位的活性位点在提高LN(OH)的光电化学水分解行为发挥了重要作用3。Ln(OH)3的稳定性在0.8 V(vs Ag / AgCl)进行计时电流法研究4小时后,对样品进行了分析,并使用X射线衍射,傅立叶变换红外光谱,拉曼光谱以及EPR和XPS分析进行了分析。结果表明,Ln(OH)3通过证明其在光电化学水分解后的相纯度表现出极好的稳定性。我们提出了Ln(OH)3作为高浓度硫化物基电解质中的高度稳定的光电化学水分解催化剂,并预期Ln(OH)3系统将被大规模探索以生产H 2作为一种生态友好的方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号