当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cyano-substituted benzochalcogenadiazole-based polymer semiconductors for balanced ambipolar organic thin-film transistors†
Polymer Chemistry ( IF 4.6 ) Pub Date : 2018-06-11 00:00:00 , DOI: 10.1039/c8py00540k Shengbin Shi 1, 2, 3, 4 , Hang Wang 1, 2, 3, 4, 5 , Peng Chen 1, 2, 3, 4 , Mohammad Afsar Uddin 6, 7, 8, 9, 10 , Yuxi Wang 1, 2, 3, 4 , Yumin Tang 1, 2, 3, 4 , Han Guo 1, 2, 3, 4 , Xing Cheng 1, 2, 3, 4 , Shiming Zhang 5, 11, 12, 13, 14 , Han Young Woo 6, 7, 8, 9, 10 , Xugang Guo 1, 2, 3, 4
Polymer Chemistry ( IF 4.6 ) Pub Date : 2018-06-11 00:00:00 , DOI: 10.1039/c8py00540k Shengbin Shi 1, 2, 3, 4 , Hang Wang 1, 2, 3, 4, 5 , Peng Chen 1, 2, 3, 4 , Mohammad Afsar Uddin 6, 7, 8, 9, 10 , Yuxi Wang 1, 2, 3, 4 , Yumin Tang 1, 2, 3, 4 , Han Guo 1, 2, 3, 4 , Xing Cheng 1, 2, 3, 4 , Shiming Zhang 5, 11, 12, 13, 14 , Han Young Woo 6, 7, 8, 9, 10 , Xugang Guo 1, 2, 3, 4
Affiliation
|
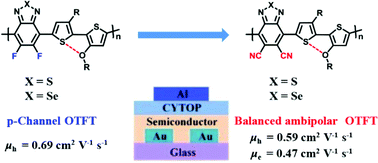
Due to their high-lying lowest unoccupied molecular orbitals (LUMOs), π-conjugated polymers based on benzothiadiazole and its derivatives typically are p-type. We report here the successful development of two narrow bandgap, ambipolar donor–acceptor copolymers, PDCNBT2T and PDCNBSe2T, which are based on new cyano-substituted strong electron acceptors, 4,7-dibromo-5,6-dicyano-2,1,3-benzothiadiazole (DCNBT) and 4,7-dibromo-5,6-dicyano-2,1,3-benzoselenadiazole (DCNBSe), respectively. Compared to their polymer analogues with fluorine substituents, the LUMO was lowered by a big margin of ca. 0.6 eV and the bandgap was reduced by 0.2–0.3 eV for the cyano-substituted polymers. Therefore, the cyano-substituted benzothiadiazole polymers showed very low-lying LUMO levels of ca. 4.3 eV. Benefiting from their narrow bandgaps of 1.1–1.2 eV and appropriately positioned LUMO levels, both polymers exhibit well balanced ambipolar transport characteristics in organic thin-film transistors, which differ from the p-type dominating transport properties of their fluorinated polymer analogues. A balanced hole/electron mobility of 0.59/0.47 cm2 V−1 s−1 was achieved for polymer PDCNBT2T, and a reduced hole/electron mobility of 0.018/0.014 cm2 V−1 s−1 was observed for the benzoselenadiazole-based PDCNBSe2T due to its lower crystallinity. These results show that the electron mobility can be enhanced by approximately two orders versus the electron mobility of the previously reported 4,7-di(thiophen-2-yl)-5,6-dicyano-2,1,3-benzothiadiazole-based polymer. This improvement was achieved by using the new acceptor units without additional electron-rich thiophene flanks, which allow a higher degree of freedom in selecting the donor co-unit and more effective tuning of energy levels of frontier molecular orbitals.
中文翻译:

用于平衡双极性有机薄膜晶体管的基于氰基取代的苯并硫属元素二唑的聚合物半导体†
基于苯并噻二唑及其衍生物的π共轭聚合物由于其高度空置的最低未占据分子轨道(LUMO),通常为p型。我们在这里报告了两种窄带隙,双极性供体-受体共聚物PDCNBT2T和PDCNBSe2T的成功开发,它们基于新的氰基取代的强电子受体4,7-dibromo-5,6-dicyano-2,1,3 -苯并噻二唑(DCNBT)和4,7-二溴-5,6-二氰基-2,1,3-苯并硒二唑(DCNBSe)。与它们的具有氟取代基的聚合物类似物相比,LUMO的降低幅度很大。对于氰基取代的聚合物,0.6 eV,带隙降低了0.2–0.3 eV。因此,氰基取代的苯并噻二唑聚合物显示出非常低的LUMO水平。4.3 eV。得益于其1.1–1.2 eV的窄带隙和适当定位的LUMO能级,这两种聚合物在有机薄膜晶体管中均表现出很好的平衡双极性传输特性,这不同于其氟化聚合物类似物的p型主导传输特性。聚合物PDCNBT2T的空穴/电子迁移率达到0.59 / 0.47 cm 2 V -1 s -1的平衡,而苯并硒二氮唑基的空穴/电子迁移率降低了0.018 / 0.014 cm 2 V -1 s -1。 PDCNBSe2T由于其较低的结晶度。这些结果表明,相对于先前报道的4,7-二(噻吩-2-基)-5,6-二氰基-2,1,3-苯并噻二唑基聚合物的电子迁移率。通过使用新的受体单元而没有额外的富电子噻吩侧翼实现了这一改进,这使选择供体共单元的自由度更高,并且更有效地调整了前沿分子轨道的能级。
更新日期:2018-06-11
中文翻译:

用于平衡双极性有机薄膜晶体管的基于氰基取代的苯并硫属元素二唑的聚合物半导体†
基于苯并噻二唑及其衍生物的π共轭聚合物由于其高度空置的最低未占据分子轨道(LUMO),通常为p型。我们在这里报告了两种窄带隙,双极性供体-受体共聚物PDCNBT2T和PDCNBSe2T的成功开发,它们基于新的氰基取代的强电子受体4,7-dibromo-5,6-dicyano-2,1,3 -苯并噻二唑(DCNBT)和4,7-二溴-5,6-二氰基-2,1,3-苯并硒二唑(DCNBSe)。与它们的具有氟取代基的聚合物类似物相比,LUMO的降低幅度很大。对于氰基取代的聚合物,0.6 eV,带隙降低了0.2–0.3 eV。因此,氰基取代的苯并噻二唑聚合物显示出非常低的LUMO水平。4.3 eV。得益于其1.1–1.2 eV的窄带隙和适当定位的LUMO能级,这两种聚合物在有机薄膜晶体管中均表现出很好的平衡双极性传输特性,这不同于其氟化聚合物类似物的p型主导传输特性。聚合物PDCNBT2T的空穴/电子迁移率达到0.59 / 0.47 cm 2 V -1 s -1的平衡,而苯并硒二氮唑基的空穴/电子迁移率降低了0.018 / 0.014 cm 2 V -1 s -1。 PDCNBSe2T由于其较低的结晶度。这些结果表明,相对于先前报道的4,7-二(噻吩-2-基)-5,6-二氰基-2,1,3-苯并噻二唑基聚合物的电子迁移率。通过使用新的受体单元而没有额外的富电子噻吩侧翼实现了这一改进,这使选择供体共单元的自由度更高,并且更有效地调整了前沿分子轨道的能级。



























 京公网安备 11010802027423号
京公网安备 11010802027423号