当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A highly active, CO2-tolerant electrode for the oxygen reduction reaction†
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2018-06-11 00:00:00 , DOI: 10.1039/c8ee01140k Yu Chen 1, 2, 3, 4 , Seonyoung Yoo 1, 2, 3, 4 , YongMan Choi 5, 6, 7 , Jun Hyuk Kim 1, 2, 3, 4 , Yong Ding 1, 2, 3, 4 , Kai Pei 1, 2, 3, 4 , Ryan Murphy 1, 2, 3, 4 , Yanxiang Zhang 1, 8, 9, 10, 11 , Bote Zhao 1, 2, 3, 4 , Weilin Zhang 1, 2, 3, 4 , Huijun Chen 12, 13, 14, 15, 16 , Yan Chen 12, 13, 14, 15, 16 , Wei Yuan 1, 2, 3, 4 , Chenghao Yang 12, 13, 14, 15, 16 , Meilin Liu 1, 2, 3, 4
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2018-06-11 00:00:00 , DOI: 10.1039/c8ee01140k Yu Chen 1, 2, 3, 4 , Seonyoung Yoo 1, 2, 3, 4 , YongMan Choi 5, 6, 7 , Jun Hyuk Kim 1, 2, 3, 4 , Yong Ding 1, 2, 3, 4 , Kai Pei 1, 2, 3, 4 , Ryan Murphy 1, 2, 3, 4 , Yanxiang Zhang 1, 8, 9, 10, 11 , Bote Zhao 1, 2, 3, 4 , Weilin Zhang 1, 2, 3, 4 , Huijun Chen 12, 13, 14, 15, 16 , Yan Chen 12, 13, 14, 15, 16 , Wei Yuan 1, 2, 3, 4 , Chenghao Yang 12, 13, 14, 15, 16 , Meilin Liu 1, 2, 3, 4
Affiliation
|
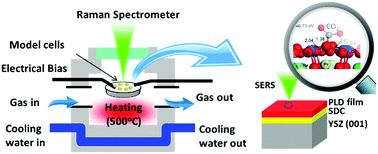
One challenge facing the development of high-performance cathodes for solid oxide fuel cells (SOFC) is the fast degradation rate of cathodes due to poisoning by contaminants commonly encountered in ambient air such as CO2. Here we report a double perovskite PrBa0.8Ca0.2Co2O5+δ (PBCC) cathode with excellent ORR activity and remarkable CO2 tolerance under realistic operation conditions. When tested in a symmetrical cell in air with ∼1 vol% CO2 at 750 °C, the PBCC electrode shows an area specific resistance of ∼0.024 Ω cm2, which increases to 0.028 Ω cm2 after 1000 h operation. The degradation rate is ∼1/24 of that of the state-of-the-art La0.6Sr0.4Co0.2Fe0.8O3 (LSCF) cathode under the same conditions. Impedance spectroscopy and in situ surface enhanced Raman spectroscopy analyses indicate that the surface of the PBCC electrode is much more active for oxygen exchange and more robust against CO2 than that of LSCF, as confirmed by density functional theory calculations. The fast ORR kinetics and excellent durability of PBCC in air with CO2 highlight the potential of PBCC as a highly promising material for devices involving oxygen electrochemistry such as solid oxide fuel cells, electrolysis cells, or gas separation membranes.
中文翻译:

用于氧还原反应的 高活性耐CO 2电极†
固体氧化物燃料电池(SOFC)高性能阴极的开发面临的一个挑战是由于环境空气中常见的污染物(例如CO 2)中毒导致的阴极快速降解。在这里,我们报道了一种双钙钛矿PrBa 0.8 Ca 0.2 Co 2 O 5+ δ(PBCC)阴极,它在实际操作条件下具有出色的ORR活性和出色的CO 2耐受性。在750°C的空气中在〜1 vol%CO 2的对称电池中测试时,PBCC电极的面积比电阻为〜0.024Ωcm 2,增加到0.028Ωcm 2在运行1000小时后。在相同条件下,其降解速率约为最先进的La 0.6 Sr 0.4 Co 0.2 Fe 0.8 O 3(LSCF)阴极的降解速率的约1.24 。阻抗谱和原位表面增强拉曼光谱分析表明,与LSCF相比,PBCC电极的表面具有更强的氧交换活性和更强的CO 2耐受性,这已得到密度泛函理论计算的证实。具有CO 2的空气中PBCC的快速ORR动力学和出色的耐久性 强调了PBCC作为涉及氧电化学的设备(如固体氧化物燃料电池,电解池或气体分离膜)的潜力巨大的潜力。
更新日期:2018-06-11
中文翻译:

用于氧还原反应的 高活性耐CO 2电极†
固体氧化物燃料电池(SOFC)高性能阴极的开发面临的一个挑战是由于环境空气中常见的污染物(例如CO 2)中毒导致的阴极快速降解。在这里,我们报道了一种双钙钛矿PrBa 0.8 Ca 0.2 Co 2 O 5+ δ(PBCC)阴极,它在实际操作条件下具有出色的ORR活性和出色的CO 2耐受性。在750°C的空气中在〜1 vol%CO 2的对称电池中测试时,PBCC电极的面积比电阻为〜0.024Ωcm 2,增加到0.028Ωcm 2在运行1000小时后。在相同条件下,其降解速率约为最先进的La 0.6 Sr 0.4 Co 0.2 Fe 0.8 O 3(LSCF)阴极的降解速率的约1.24 。阻抗谱和原位表面增强拉曼光谱分析表明,与LSCF相比,PBCC电极的表面具有更强的氧交换活性和更强的CO 2耐受性,这已得到密度泛函理论计算的证实。具有CO 2的空气中PBCC的快速ORR动力学和出色的耐久性 强调了PBCC作为涉及氧电化学的设备(如固体氧化物燃料电池,电解池或气体分离膜)的潜力巨大的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号