当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dual Intratumoral Redox/Enzyme‐Responsive NO‐Releasing Nanomedicine for the Specific, High‐Efficacy, and Low‐Toxic Cancer Therapy
Advanced Materials ( IF 29.4 ) Pub Date : 2018-06-11 , DOI: 10.1002/adma.201704490 Xiaobo Jia 1 , Yihua Zhang 2 , Yu Zou 2 , Yao Wang 1 , Dechao Niu 1 , Qianjun He 3 , Zhangjian Huang 2 , Weihong Zhu 4 , He Tian 4 , Jianlin Shi 1 , Yongsheng Li 1
Advanced Materials ( IF 29.4 ) Pub Date : 2018-06-11 , DOI: 10.1002/adma.201704490 Xiaobo Jia 1 , Yihua Zhang 2 , Yu Zou 2 , Yao Wang 1 , Dechao Niu 1 , Qianjun He 3 , Zhangjian Huang 2 , Weihong Zhu 4 , He Tian 4 , Jianlin Shi 1 , Yongsheng Li 1
Affiliation
|
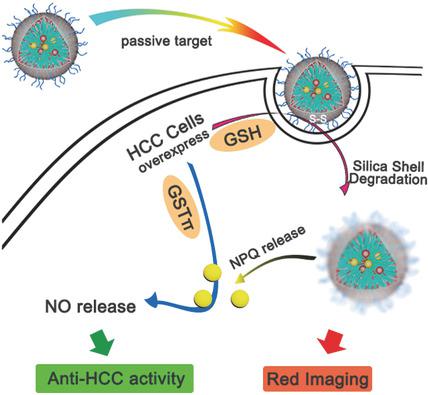
Chemotherapy suffers numbers of limitations including poor drug solubility, nonspecific biodistribution, and inevitable adverse effects on normal tissues. Tumor‐targeted delivery and intratumoral stimuli‐responsive release of drugs by nanomedicines are considered to be highly promising in solving these problems. Compared with traditional chemotherapeutic drugs, high concentration of nitric oxide (NO) exhibits unique anticancer effects. The development of tumor‐targeting and intratumoral microenvironment‐responsive NO‐releasing nanomedicines is highly desired. Here a novel kind of organic–inorganic composite nanomedicine (QM‐NPQ@PDHNs) is presented by encapsulating a glutathione S‐transferases π (GSTπ)‐responsive drug O2‐(2,4‐dinitro‐5‐{[2‐(β‐d‐galactopyranosyl olean‐12‐en‐28‐oate‐3‐yl)‐oxy‐2‐oxoethyl] piperazine‐1‐yl} phenyl) 1‐(methylethanolamino)diazen‐1‐ium‐1,2‐dilate (NPQ) as NO donor and an aggregation‐induced‐emission (AIE) red fluorogen QM‐2 into the cores of the hybrid nanomicelles (PEGylated disulfide‐doped hybrid nanocarriers (PDHNs)) with glutathione (GSH)‐responsive shells. The QM‐NPQ@PDHN nanomedicine is able to respond to the intratumoral over‐expressed GSH and GSTπ, resulting in the responsive biodegradation of the protective organosilica shell and NPQ release, and subsequent NO release within the tumor, respectively, and thus normal organs remain unaffected. This work demonstrates a paradigm of dual intratumoral redox/enzyme‐responsive NO‐release nanomedicine for tumor‐specific and high‐efficacy cancer therapy.
中文翻译:

双重肿瘤内氧化还原/酶响应性NO释放纳米药物,用于特异性,高效和低毒的癌症治疗
化学疗法受到许多限制,包括药物溶解性差,非特异性生物分布以及对正常组织不可避免的不利影响。纳米药物对肿瘤的靶向递送和肿瘤内刺激响应释放被认为在解决这些问题方面非常有前途。与传统的化疗药物相比,高浓度的一氧化氮(NO)表现出独特的抗癌作用。迫切需要开发靶向肿瘤和瘤内微环境反应性的可释放NO的纳米药物。本文通过包封谷胱甘肽S转移酶π(GSTπ)反应性药物O 2-(2,4-dinitro-5-{[2-( β- d-吡喃半乳糖基油基羊毛基-12-en-28-oate-3-基)-氧-2-氧代乙基]哌嗪-1-基}苯基)1-(甲基乙醇氨基)重氮-1-基1,2-二磺酸盐(NPQ)作为NO供体和聚集诱导排放(AIE)的红色荧光素QM-2进入具有谷胱甘肽(GSH)响应壳的杂化纳米胶束(PEG化二硫掺杂杂化纳米载体(PDHNs))的核中。QM‐NPQ @ PDHN纳米药物能够对肿瘤内过表达的GSH和GSTπ作出反应,从而导致保护性有机硅壳的响应性生物降解和NPQ释放,以及随后在肿瘤内的NO释放,因此正常器官得以保留。不受影响。这项工作展示了双重肿瘤内氧化还原/酶反应性NO释放纳米药物用于肿瘤特异性和高效率癌症治疗的范例。
更新日期:2018-06-11
中文翻译:

双重肿瘤内氧化还原/酶响应性NO释放纳米药物,用于特异性,高效和低毒的癌症治疗
化学疗法受到许多限制,包括药物溶解性差,非特异性生物分布以及对正常组织不可避免的不利影响。纳米药物对肿瘤的靶向递送和肿瘤内刺激响应释放被认为在解决这些问题方面非常有前途。与传统的化疗药物相比,高浓度的一氧化氮(NO)表现出独特的抗癌作用。迫切需要开发靶向肿瘤和瘤内微环境反应性的可释放NO的纳米药物。本文通过包封谷胱甘肽S转移酶π(GSTπ)反应性药物O 2-(2,4-dinitro-5-{[2-( β- d-吡喃半乳糖基油基羊毛基-12-en-28-oate-3-基)-氧-2-氧代乙基]哌嗪-1-基}苯基)1-(甲基乙醇氨基)重氮-1-基1,2-二磺酸盐(NPQ)作为NO供体和聚集诱导排放(AIE)的红色荧光素QM-2进入具有谷胱甘肽(GSH)响应壳的杂化纳米胶束(PEG化二硫掺杂杂化纳米载体(PDHNs))的核中。QM‐NPQ @ PDHN纳米药物能够对肿瘤内过表达的GSH和GSTπ作出反应,从而导致保护性有机硅壳的响应性生物降解和NPQ释放,以及随后在肿瘤内的NO释放,因此正常器官得以保留。不受影响。这项工作展示了双重肿瘤内氧化还原/酶反应性NO释放纳米药物用于肿瘤特异性和高效率癌症治疗的范例。


























 京公网安备 11010802027423号
京公网安备 11010802027423号