当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Secondary and Tertiary Alkyl Boronic Esters by gem‐Carboborylation: Carbonyl Compounds as Bis(electrophile) Equivalents
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-07-12 , DOI: 10.1002/anie.201804684 Dunfa Shi 1, 2 , Lu Wang 1 , Chungu Xia 1 , Chao Liu 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-07-12 , DOI: 10.1002/anie.201804684 Dunfa Shi 1, 2 , Lu Wang 1 , Chungu Xia 1 , Chao Liu 1
Affiliation
|
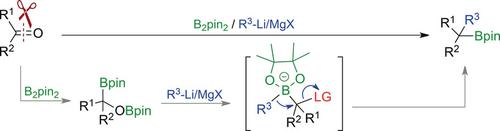
An unprecedent gem‐carboborylation of aldehydes and ketones provides access to various secondary and tertiary alkyl boronic esters. The addition of B2pin2 to a carbonyl compound generates α‐oxyl‐substituted alkyl boron species. Organolithium and Grignard reagents are then applied as C nucleophiles for the 1,2‐metalate rearrangement process. The organolithium reagents can also be generated by C−H lithiation or halogen/lithium exchange. The use of chiral ligands led to the generation of chiral alkyl boronic esters in enantioenriched form, demonstrating that the enantioselectivity of this transformation is catalyst‐controlled.
中文翻译:

宝石-碳硼化反应合成仲和叔烷基硼酸酯:羰基化合物的双(亲电子基)当量
醛和酮具有前所未有的宝石碳羰基化作用,可以使用各种仲和叔烷基硼酸酯。将B 2 pin 2加到羰基化合物上可生成α-氧基取代的烷基硼。然后将有机锂和格氏试剂用作C-亲核试剂,用于1,2-金属酸盐重排过程。有机锂试剂也可以通过CH锂化或卤素/锂交换生成。手性配体的使用导致了对映体富集形式的手性烷基硼酸酯的生成,表明这种转化的对映选择性是由催化剂控制的。
更新日期:2018-07-12
中文翻译:

宝石-碳硼化反应合成仲和叔烷基硼酸酯:羰基化合物的双(亲电子基)当量
醛和酮具有前所未有的宝石碳羰基化作用,可以使用各种仲和叔烷基硼酸酯。将B 2 pin 2加到羰基化合物上可生成α-氧基取代的烷基硼。然后将有机锂和格氏试剂用作C-亲核试剂,用于1,2-金属酸盐重排过程。有机锂试剂也可以通过CH锂化或卤素/锂交换生成。手性配体的使用导致了对映体富集形式的手性烷基硼酸酯的生成,表明这种转化的对映选择性是由催化剂控制的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号