当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Studies of the Cu(OH)+‐Catalyzed Isomerization of Glucose into Fructose in Water
ChemSusChem ( IF 8.4 ) Pub Date : 2018-07-10 , DOI: 10.1002/cssc.201800483 Joel B. Mensah 1 , Irina Delidovich 1 , Peter J. C. Hausoul 1 , Laurent Weisgerber 1 , Wolfgang Schrader 2 , Regina Palkovits 1
ChemSusChem ( IF 8.4 ) Pub Date : 2018-07-10 , DOI: 10.1002/cssc.201800483 Joel B. Mensah 1 , Irina Delidovich 1 , Peter J. C. Hausoul 1 , Laurent Weisgerber 1 , Wolfgang Schrader 2 , Regina Palkovits 1
Affiliation
|
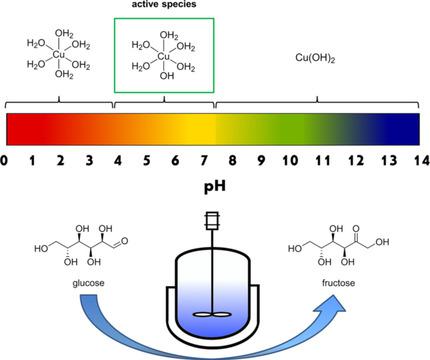
The isomerization of glucose to fructose is a crucial interim step in the processing of biomass to renewable fuels and chemicals. This study investigates the copper‐catalyzed glucose–fructose isomerization in water, focusing on insights into the roles of the dissolved copper species. Depending on the pH, the thermodynamic equilibrium shifted towards one or a few copper species, namely Cu2+, Cu(OH)+, and Cu(OH)2. According to thermodynamics, the highest concentration of Cu(OH)+ is at pH 5.3, at which the highest fructose yield of 16 % is achieved. The obtained fructose yields strongly correlate with the concentration of Cu(OH)+. A pH decrease of 2–3 units was observed during the reaction, resulting in the deactivation of the catalyst through hydrolysis in acidic media. Based on the results of the catalytic experiments, as well as spectroscopic and spectrometric studies, we propose Cu(OH)+ as an active Lewis‐acidic species following an intramolecular 1,2‐hydride shift.
中文翻译:

Cu(OH)+催化葡萄糖在水中异构化为果糖的机理研究
葡萄糖异构化为果糖是将生物质加工成可再生燃料和化学品的关键过渡步骤。这项研究调查了铜催化的葡萄糖-果糖在水中的异构化,重点是对溶解的铜物质的作用的见解。取决于pH,热力学平衡向一种或几种铜物质,即Cu 2 +,Cu(OH)+和Cu(OH)2转移。根据热力学,Cu(OH)+的最高浓度为pH 5.3,在该pH下,果糖的最高收率为16%。获得的果糖产量与Cu(OH)+的浓度密切相关。反应过程中观察到pH降低了2-3个单位,导致催化剂在酸性介质中水解而失活。基于催化实验的结果,以及光谱和光谱研究,我们建议将Cu(OH)+作为分子内1,2-氢化物转移后的活性Lewis酸性物质。
更新日期:2018-07-10
中文翻译:

Cu(OH)+催化葡萄糖在水中异构化为果糖的机理研究
葡萄糖异构化为果糖是将生物质加工成可再生燃料和化学品的关键过渡步骤。这项研究调查了铜催化的葡萄糖-果糖在水中的异构化,重点是对溶解的铜物质的作用的见解。取决于pH,热力学平衡向一种或几种铜物质,即Cu 2 +,Cu(OH)+和Cu(OH)2转移。根据热力学,Cu(OH)+的最高浓度为pH 5.3,在该pH下,果糖的最高收率为16%。获得的果糖产量与Cu(OH)+的浓度密切相关。反应过程中观察到pH降低了2-3个单位,导致催化剂在酸性介质中水解而失活。基于催化实验的结果,以及光谱和光谱研究,我们建议将Cu(OH)+作为分子内1,2-氢化物转移后的活性Lewis酸性物质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号