当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Charge Environment and Hydrogen Bond Dynamics in Binary Ionic Liquid Mixtures: A Computational Study
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1021/acs.jpclett.8b01481 Nikhil V. S. Avula 1 , Anirban Mondal 1 , Sundaram Balasubramanian 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1021/acs.jpclett.8b01481 Nikhil V. S. Avula 1 , Anirban Mondal 1 , Sundaram Balasubramanian 1
Affiliation
|
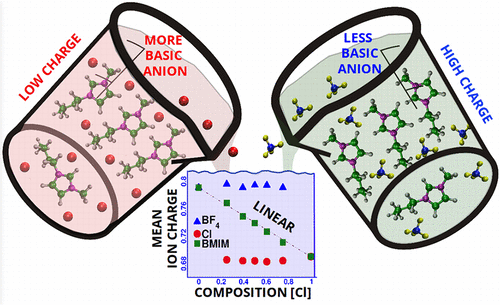
The extent of charge transfer between the cation and the anion in a room-temperature ionic liquid depends on the basicity of the anion. Ion charges determined in the condensed state via density functional theory calculations capture this effect rather well, and charges derived in such a manner have been employed in force field-based molecular dynamics simulations to quantitatively reproduce several physical properties of the liquids. However, the issue of transferability of cation charges in mixtures of ionic liquids, say with one type of cation and two different anion types needs to be addressed. Herein, we demonstrate that the cation charge in such a mixture varies linearly with anion composition, a result that ties in rather well with X-ray photoelectron spectroscopic experiments. The variation in cation charge with bulk anion composition is shown to be a result of changes in its coordination environment. Cations surrounded by a higher proportion of more basic anions possess lower charges than those surrounded by less basic anions. Time scales for the exchange of anion types for the occupation of hydrogen bonding sites around the cation have been determined and are seen to be constituted by three processes–breakage of existing hydrogen bond, diffusion to the hydrogen bonding site and displacement of the incumbent anion from its site in the cation coordination shell. These time scales explain the differences observed between infrared and NMR spectroscopic experiments in ionic liquid mixtures rather well.
中文翻译:

二元离子液体混合物中的电荷环境和氢键动力学:计算研究
室温离子液体中阳离子和阴离子之间的电荷转移程度取决于阴离子的碱性。通过密度泛函理论计算在冷凝状态下确定的离子电荷相当好地捕获了这种效应,并且以这种方式导出的电荷已被用于基于力场的分子动力学模拟中,以定量地再现液体的几种物理性质。然而,需要解决阳离子液体在离子液体混合物中的转移性问题,例如一种阳离子类型和两种不同阴离子类型。在本文中,我们证明了这种混合物中的阳离子电荷随阴离子组成线性变化,这一结果与X射线光电子能谱实验相吻合。阳离子电荷随整体阴离子组成的变化被证明是其配位环境变化的结果。与被较少碱性阴离子所包围的阳离子相比,被较高碱性阴离子所占比例较高的阳离子所具有的电荷较低。已经确定了阴离子类型交换占据阳离子周围氢键位的时间尺度,并认为这是由三个过程构成的:现有氢键的断裂,向氢键位的扩散以及现有阴离子从中的置换。它的位置在阳离子配位壳中。这些时间尺度很好地解释了离子液体混合物中红外光谱和NMR光谱实验之间观察到的差异。与被较少碱性阴离子所包围的阳离子相比,被较高碱性阴离子所占比例较高的阳离子所具有的电荷较低。已经确定了阴离子类型交换占据阳离子周围氢键位的时间尺度,并认为这是由三个过程构成的:现有氢键的断裂,向氢键位的扩散以及现有阴离子从中的置换。它的位置在阳离子配位壳中。这些时间尺度很好地解释了离子液体混合物中红外光谱和NMR光谱实验之间观察到的差异。与被较少碱性阴离子包围的阳离子相比,被较多碱性阴离子包围的阳离子具有较低的电荷。已经确定了阴离子类型交换占据阳离子周围氢键位的时间尺度,并认为这是由三个过程构成的:现有氢键的断裂,向氢键位的扩散以及现有阴离子从中的置换。它的位置在阳离子配位壳中。这些时间尺度很好地解释了离子液体混合物中红外光谱和NMR光谱实验之间观察到的差异。已经确定了阴离子类型交换占据阳离子周围氢键位的时间尺度,并认为这是由三个过程组成的:现有氢键的断裂,向氢键位的扩散以及现有阴离子从中的置换。它的位置在阳离子配位壳中。这些时间尺度很好地解释了离子液体混合物中红外光谱和NMR光谱实验之间观察到的差异。已经确定了阴离子类型交换占据阳离子周围氢键位的时间尺度,并认为这是由三个过程构成的:现有氢键的断裂,向氢键位的扩散以及现有阴离子从中的置换。它的位置在阳离子配位壳中。这些时间尺度很好地解释了离子液体混合物中红外光谱和NMR光谱实验之间观察到的差异。
更新日期:2018-06-08
中文翻译:

二元离子液体混合物中的电荷环境和氢键动力学:计算研究
室温离子液体中阳离子和阴离子之间的电荷转移程度取决于阴离子的碱性。通过密度泛函理论计算在冷凝状态下确定的离子电荷相当好地捕获了这种效应,并且以这种方式导出的电荷已被用于基于力场的分子动力学模拟中,以定量地再现液体的几种物理性质。然而,需要解决阳离子液体在离子液体混合物中的转移性问题,例如一种阳离子类型和两种不同阴离子类型。在本文中,我们证明了这种混合物中的阳离子电荷随阴离子组成线性变化,这一结果与X射线光电子能谱实验相吻合。阳离子电荷随整体阴离子组成的变化被证明是其配位环境变化的结果。与被较少碱性阴离子所包围的阳离子相比,被较高碱性阴离子所占比例较高的阳离子所具有的电荷较低。已经确定了阴离子类型交换占据阳离子周围氢键位的时间尺度,并认为这是由三个过程构成的:现有氢键的断裂,向氢键位的扩散以及现有阴离子从中的置换。它的位置在阳离子配位壳中。这些时间尺度很好地解释了离子液体混合物中红外光谱和NMR光谱实验之间观察到的差异。与被较少碱性阴离子所包围的阳离子相比,被较高碱性阴离子所占比例较高的阳离子所具有的电荷较低。已经确定了阴离子类型交换占据阳离子周围氢键位的时间尺度,并认为这是由三个过程构成的:现有氢键的断裂,向氢键位的扩散以及现有阴离子从中的置换。它的位置在阳离子配位壳中。这些时间尺度很好地解释了离子液体混合物中红外光谱和NMR光谱实验之间观察到的差异。与被较少碱性阴离子包围的阳离子相比,被较多碱性阴离子包围的阳离子具有较低的电荷。已经确定了阴离子类型交换占据阳离子周围氢键位的时间尺度,并认为这是由三个过程构成的:现有氢键的断裂,向氢键位的扩散以及现有阴离子从中的置换。它的位置在阳离子配位壳中。这些时间尺度很好地解释了离子液体混合物中红外光谱和NMR光谱实验之间观察到的差异。已经确定了阴离子类型交换占据阳离子周围氢键位的时间尺度,并认为这是由三个过程组成的:现有氢键的断裂,向氢键位的扩散以及现有阴离子从中的置换。它的位置在阳离子配位壳中。这些时间尺度很好地解释了离子液体混合物中红外光谱和NMR光谱实验之间观察到的差异。已经确定了阴离子类型交换占据阳离子周围氢键位的时间尺度,并认为这是由三个过程构成的:现有氢键的断裂,向氢键位的扩散以及现有阴离子从中的置换。它的位置在阳离子配位壳中。这些时间尺度很好地解释了离子液体混合物中红外光谱和NMR光谱实验之间观察到的差异。



























 京公网安备 11010802027423号
京公网安备 11010802027423号