Journal of Colloid and Interface Science ( IF 9.9 ) Pub Date : 2018-06-08 , DOI: 10.1016/j.jcis.2018.06.019 V.M. Gun'ko , E.M. Pakhlov , O.V. Goncharuk , L.S. Andriyko , Yu.M. Nychiporuk , D.Yu. Balakin , D. Sternik , A. Derylo-Marczewska
|
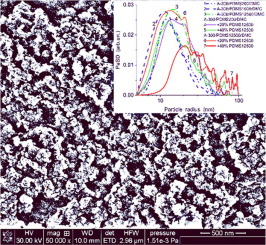
Three polydimethylsiloxanes (PDMS200, PDMS1000, and PDMS12500 with numbers showing the viscosity values dependent on the molecular weight) were used for adsorption (14–95 wt% PDMS) onto unmodified and PDMS-modified (16.7 wt% PDMS using dimethyl carbonate (DMC) as a siloxane bond breaking reagent) nanosilica A-300. The materials were studied using microscopy, infrared spectroscopy, thermodesorption, calorimetry, ethanol and water/ethanol evaporation, nitrogen adsorption-desorption, and quantum chemical methods. The interfacial and temperature behaviors of a PDMS layer at a silica surface depend strongly on the type of bonding to silica particles, molecular weight and content of PDMS. Upon chemical bonding, shorter PDMS200 forms a denser coverage of the silica surface since SBET diminution is larger and residual free silanols are practically absent (the degree of free silanol substitution Θ > 0.95) in contrast to the reactions with PDMS1000/DMC or PDMS12500/DMC providing Θ = 0.60–0.63 at larger SBET values. Upon thermal decomposition of the PDMS layer, oxidation/depolymerization desorption gives a greater contribution than pure depolymerization destruction. An increase in the PDMS adsorption layer thickness leads to enhancement of the depolymerization contribution because the oxidation mainly occurs at the top of the layer, but the depolymerization can occur in the total PDMS layer. The adsorption, desorption, and evaporation processes of low-molecular weight probes at a surface of PDMS-modified nanosilica depend strongly on the type of bonding and content of PDMS. Thus, the most effective hydrophobization of nanosilica by PDMS/DMC could be carried out using the shortest polymer giving the shortest PDMS fragments upon the interaction with DMC that is of interest from a practical point of view.
中文翻译:

聚二甲基硅氧烷改性的纳米二氧化硅解聚并化学键合到纳米颗粒上或物理键合到未修饰或修饰的表面上:结构和界面现象
三种聚二甲基硅氧烷(PDMS200,PDMS1000和PDMS12500,其数字表示粘度值取决于分子量)被用于吸附(14–95 wt%PDMS)到未改性和PDMS改性(使用碳酸二甲酯(DMC)的16.7 wt%PDMS)上作为硅氧烷键断裂试剂)纳米二氧化硅A-300。使用显微镜,红外光谱,热脱附,量热法,乙醇和水/乙醇蒸发,氮吸附-脱附以及量子化学方法对材料进行了研究。二氧化硅表面上的PDMS层的界面和温度行为在很大程度上取决于与二氧化硅颗粒的键合类型,分子量和PDMS的含量。经过化学键合后,较短的PDMS200由于S BET而形成更紧密的二氧化硅表面覆盖与PDMS1000 / DMC或PDMS12500 / DMC的反应在较大的S BET下提供Θ= 0.60–0.63的反应相比,减小量更大并且几乎没有残留的游离硅烷醇(游离硅烷醇取代度Θ> 0.95)价值观。在PDMS层热分解后,氧化/解聚解吸作用比纯解聚破坏作用更大。PDMS吸附层厚度的增加导致解聚作用的增强,因为氧化主要发生在层的顶部,但是解聚可以发生在整个PDMS层中。低分子量探针在PDMS改性的纳米二氧化硅表面的吸附,解吸和蒸发过程在很大程度上取决于键合的类型和PDMS的含量。因此,可以使用PDMS / DMC对纳米二氧化硅进行最有效的疏水化,这是使用最短的聚合物,该聚合物在与DMC相互作用时给出最短的PDMS片段,这在实践中是有意义的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号