当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electron Spin Resonance Study of Molecular Orientation and Dynamics of Phenyl Imino and Nitronyl Nitroxide Radicals in Organic 1D Nanochannels of Tris(o-phenylenedioxy)cyclotriphosphazene
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-06-07 00:00:00 , DOI: 10.1021/acs.jpca.8b01806 Hirokazu Kobayashi 1 , Takanori Mori 2 , Yuka Morinaga 2 , Etsuko Fujimori 2 , Kento Akiniwa 3 , Fumiyasu Iwahori 2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-06-07 00:00:00 , DOI: 10.1021/acs.jpca.8b01806 Hirokazu Kobayashi 1 , Takanori Mori 2 , Yuka Morinaga 2 , Etsuko Fujimori 2 , Kento Akiniwa 3 , Fumiyasu Iwahori 2
Affiliation
|
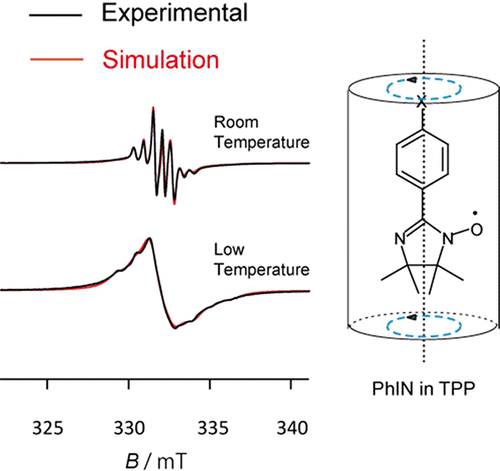
Imino and nitronyl nitroxide (IN and NN, respectively) radicals such as phenyliminonitroxide (PhIN) and phenylnitronylnitroxide (PhNN), respectively, were dispersed in the organic 1D nanochannels of tris(o-phenylenedioxy)cyclotriphosphazene (TPP). Electron spin resonance (ESR) measurements were conducted on these inclusion compounds (ICs) in the temperature range 4.2–300 K. The modulated-septet ESR spectra of TPP ICs using PhIN observed in the range 165–258 K were reproduced with the EasySpin program package using a model in which some of the PhIN molecules underwent uniaxial rotational diffusion in the TPP nanochannels around the molecular long axis corresponding to the principal y-axis of the g tensor. However, for the TPP IC using PhNN, complicated ESR spectra were observed, which were not consistent with the modulated quintet observed in solution or in the fast-motion limit in solids. These spectra were reproduced by the superposition of a quintet originating from rotational diffusion of PhNN molecules and a septet based on rotational diffusion of PhIN molecules generated in the synthetic process. The rotational diffusion activation energies of PhIN and PhNN in the TPP nanochannels were estimated to be 19 and 45 kJ mol–1 using an Arrhenius plot, respectively. These were consistent with that for NN radicals in the 1D nanochannels of 2,4,6-tris(4-chlorophenoxy)-1,3,5-triazine (CLPOT) (37–54 kJ mol–1) with a larger pore diameter than TPP reported in our previous study, or with that for 4-substituted-2,2,6,6-tetramethyl-1-piperidinyloxyl (4-X-TEMPO) in TPP nanochannels (5–26 kJ mol–1) with regard to molecular size or host–guest or guest–guest interactions. These results indicate that not only the NN group but also IN may be used for the clarification of chemical or biological structures of nanomaterials such as nanosized cavities.
中文翻译:

三(邻苯二甲氧基)环三磷腈有机一维纳米通道中苯基亚氨基和一氧化氮氮自由基的分子取向和动力学的电子自旋共振研究
分别将亚氨基硝酰基和亚硝酰基硝基(IN和NN)自由基(例如苯基亚氨基硝基氧(PhIN)和苯基亚硝酰基硝基氧(PhNN))分散在三(邻苯二甲氧基)环三磷腈(TPP)的有机一维纳米通道中。在4.2–300 K的温度范围内对这些夹杂物(IC)进行了电子自旋共振(ESR)测量。使用EasySpin程序重现了使用PhIN在165–258 K范围内观察到的TPP IC的调制肽段ESR谱图。使用以下模型进行包装:模型中,一些PhIN分子在TPP纳米通道中围绕与g的主要y轴相对应的分子长轴进行了单轴旋转扩散张量。但是,对于使用PhNN的TPP IC,观察到了复杂的ESR光谱,这与在溶液中或在固体中的快速运动极限中观察到的调制五重态不一致。这些光谱是通过叠加源自PhNN分子旋转扩散的五重音和基于合成过程中生成的PhIN分子旋转扩散的音序音的叠加而重现的。使用Arrhenius曲线估计,TPP纳米通道中PhIN和PhNN的旋转扩散活化能分别为19和45 kJ mol –1。这些与2,4,6-tris(4-chlorophenoxy)-1,3,5-triazine(CLPOT)(37–54 kJ mol –1)的孔径大于我们先前研究中报告的TPP,或在TPP纳米通道中(5-26 kJ)具有4-取代的2,2,6,6-四甲基-1-哌啶基氧基(4-X-TEMPO)的孔径mol –1)关于分子大小或主客体或客客体相互作用。这些结果表明,不仅NN基团,而且IN也可用于澄清纳米材料例如纳米腔的化学或生物学结构。
更新日期:2018-06-07
中文翻译:

三(邻苯二甲氧基)环三磷腈有机一维纳米通道中苯基亚氨基和一氧化氮氮自由基的分子取向和动力学的电子自旋共振研究
分别将亚氨基硝酰基和亚硝酰基硝基(IN和NN)自由基(例如苯基亚氨基硝基氧(PhIN)和苯基亚硝酰基硝基氧(PhNN))分散在三(邻苯二甲氧基)环三磷腈(TPP)的有机一维纳米通道中。在4.2–300 K的温度范围内对这些夹杂物(IC)进行了电子自旋共振(ESR)测量。使用EasySpin程序重现了使用PhIN在165–258 K范围内观察到的TPP IC的调制肽段ESR谱图。使用以下模型进行包装:模型中,一些PhIN分子在TPP纳米通道中围绕与g的主要y轴相对应的分子长轴进行了单轴旋转扩散张量。但是,对于使用PhNN的TPP IC,观察到了复杂的ESR光谱,这与在溶液中或在固体中的快速运动极限中观察到的调制五重态不一致。这些光谱是通过叠加源自PhNN分子旋转扩散的五重音和基于合成过程中生成的PhIN分子旋转扩散的音序音的叠加而重现的。使用Arrhenius曲线估计,TPP纳米通道中PhIN和PhNN的旋转扩散活化能分别为19和45 kJ mol –1。这些与2,4,6-tris(4-chlorophenoxy)-1,3,5-triazine(CLPOT)(37–54 kJ mol –1)的孔径大于我们先前研究中报告的TPP,或在TPP纳米通道中(5-26 kJ)具有4-取代的2,2,6,6-四甲基-1-哌啶基氧基(4-X-TEMPO)的孔径mol –1)关于分子大小或主客体或客客体相互作用。这些结果表明,不仅NN基团,而且IN也可用于澄清纳米材料例如纳米腔的化学或生物学结构。


























 京公网安备 11010802027423号
京公网安备 11010802027423号