当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phase Equilibria in the Ternary System CaCl2–SrCl2–H2O and the Quaternary System KCl–CaCl2–SrCl2–H2O at 373 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-06-07 , DOI: 10.1021/acs.jced.8b00087 Yun-Yun Gao 1 , Chao Ye 1 , Wen-Yao Zhang 1 , Lei Yang 1 , Shi-Hua Sang 1, 2 , Yi Huang 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-06-07 , DOI: 10.1021/acs.jced.8b00087 Yun-Yun Gao 1 , Chao Ye 1 , Wen-Yao Zhang 1 , Lei Yang 1 , Shi-Hua Sang 1, 2 , Yi Huang 1
Affiliation
|
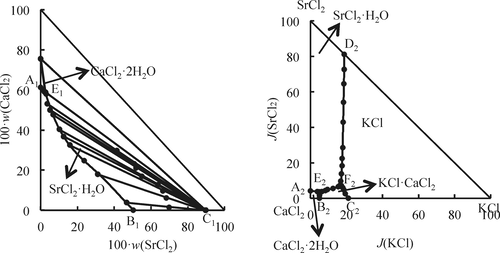
The stable phase equilibria of the ternary system CaCl2–SrCl2–H2O and the quaternary system KCl–CaCl2–SrCl2–H2O at 373 K were investigated by means of the isothermal dissolution equilibrium method. According to experimental results, the phase diagrams of the two systems were plotted. The results show that the ternary system CaCl2–SrCl2–H2O at 373 K belongs to the simple cosaturated type, without complex salt or solid solution. There are one invariant point, two univariant curves, and two crystallization fields (where the solids are SrCl2·H2O and CaCl2·2H2O). The quaternary system KCl–CaCl2–SrCl2–H2O at 373 K has a double salt, chlorocalcite (KCl·CaCl2). The phase diagram contains two invariant points, five univariant curves, and four crystallization fields (where the solids are KCl, CaCl2·2H2O, SrCl2·H2O, and KCl·CaCl2).
中文翻译:

CaCl 2 -SrCl 2 -H 2 O三元体系和KCl-CaCl 2 -SrCl 2 -H 2 O四元体系在373 K时的相平衡
采用等温溶出平衡法研究了三元系CaCl 2 -SrCl 2 -H 2 O和四元系KCl-CaCl 2 -SrCl 2 -H 2 O在373 K时的稳定相平衡。根据实验结果,绘制了两个系统的相图。结果表明,三元体系CaCl 2 -SrCl 2 -H 2 O在373 K时属于简单共饱和类型,没有复杂的盐或固溶体。有一个不变点,两个单变曲线和两个结晶场(其中固体为SrCl 2 ·H 2 O和CaCl2 ·2H 2 O)。373 K的四元体系KCl–CaCl 2 –SrCl 2 –H 2 O具有复盐,即氯方解石(KCl·CaCl 2)。相图包含两个不变点,五个单变量曲线和四个结晶场(其中固体为KCl,CaCl 2 ·2H 2 O,SrCl 2 ·H 2 O和KCl·CaCl 2)。
更新日期:2018-06-07
中文翻译:

CaCl 2 -SrCl 2 -H 2 O三元体系和KCl-CaCl 2 -SrCl 2 -H 2 O四元体系在373 K时的相平衡
采用等温溶出平衡法研究了三元系CaCl 2 -SrCl 2 -H 2 O和四元系KCl-CaCl 2 -SrCl 2 -H 2 O在373 K时的稳定相平衡。根据实验结果,绘制了两个系统的相图。结果表明,三元体系CaCl 2 -SrCl 2 -H 2 O在373 K时属于简单共饱和类型,没有复杂的盐或固溶体。有一个不变点,两个单变曲线和两个结晶场(其中固体为SrCl 2 ·H 2 O和CaCl2 ·2H 2 O)。373 K的四元体系KCl–CaCl 2 –SrCl 2 –H 2 O具有复盐,即氯方解石(KCl·CaCl 2)。相图包含两个不变点,五个单变量曲线和四个结晶场(其中固体为KCl,CaCl 2 ·2H 2 O,SrCl 2 ·H 2 O和KCl·CaCl 2)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号