当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
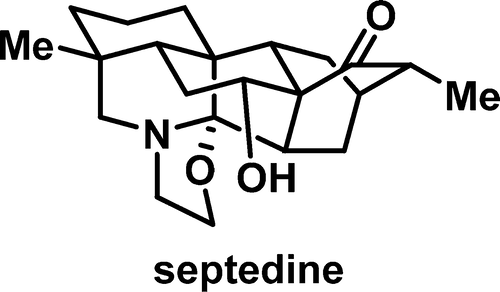
Total Synthesis of Septedine and 7-Deoxyseptedine
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-06-06 , DOI: 10.1021/jacs.8b03712 Shupeng Zhou 1 , Rui Guo 1 , Peng Yang 1 , Ang Li 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-06-06 , DOI: 10.1021/jacs.8b03712 Shupeng Zhou 1 , Rui Guo 1 , Peng Yang 1 , Ang Li 1
Affiliation
|
Septedine (2) is a hetidine type C20-diterpenoid alkaloid bearing an oxygenated heptacyclic scaffold. We have accomplished the first and asymmetric total synthesis of 2 and its 7-deoxy analogue 3. A functionalized tricyclic intermediate was prepared with excellent enantiopurity by using Carreira polyene cyclization. An unusual anionic Diels-Alder reaction was responsible for the construction of the bicyclo[2.2.2]octane. The α-methyl ketone was furnished by iridium-catalyzed allylic alcohol isomerization. Sanford Csp3-H oxidation was exploited to install the secondary hydroxy group of 2. The oxazolidinopiperidine was assembled by selective reductive amination and spontaneous N, O-ketalization at a final stage.
中文翻译:

Septedine 和 7-Deoxyseptedine 的全合成
Septedine (2) 是一种带有氧化七环支架的杂丁酸 C20-二萜生物碱。我们首次完成了2及其7-脱氧类似物3的不对称全合成。利用Carreira多烯环化制备了具有优异对映纯度的功能化三环中间体。一个不寻常的阴离子 Diels-Alder 反应负责构建双环 [2.2.2] 辛烷。α-甲基酮通过铱催化的烯丙醇异构化得到。利用 Sanford Csp3-H 氧化来安装 2 的仲羟基。最后阶段通过选择性还原胺化和自发 N, O-缩酮组装恶唑烷并哌啶。
更新日期:2018-06-06
中文翻译:

Septedine 和 7-Deoxyseptedine 的全合成
Septedine (2) 是一种带有氧化七环支架的杂丁酸 C20-二萜生物碱。我们首次完成了2及其7-脱氧类似物3的不对称全合成。利用Carreira多烯环化制备了具有优异对映纯度的功能化三环中间体。一个不寻常的阴离子 Diels-Alder 反应负责构建双环 [2.2.2] 辛烷。α-甲基酮通过铱催化的烯丙醇异构化得到。利用 Sanford Csp3-H 氧化来安装 2 的仲羟基。最后阶段通过选择性还原胺化和自发 N, O-缩酮组装恶唑烷并哌啶。



























 京公网安备 11010802027423号
京公网安备 11010802027423号