当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Revealing the unusual role of bases in activation/deactivation of catalytic systems: O–NHC coupling in M/NHC catalysis†
Chemical Science ( IF 8.4 ) Pub Date : 2018-06-06 00:00:00 , DOI: 10.1039/c8sc01353e Victor M. Chernyshev 1, 2, 3 , Oleg V. Khazipov 1, 2, 3 , Maxim A. Shevchenko 1, 2, 3 , Andrey Yu. Chernenko 1, 2, 3 , Alexander V. Astakhov 1, 2, 3 , Dmitry B. Eremin 3, 4, 5, 6 , Dmitry V. Pasyukov 1, 2, 3 , Alexey S. Kashin 3, 4, 5, 6 , Valentine P. Ananikov 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2018-06-06 00:00:00 , DOI: 10.1039/c8sc01353e Victor M. Chernyshev 1, 2, 3 , Oleg V. Khazipov 1, 2, 3 , Maxim A. Shevchenko 1, 2, 3 , Andrey Yu. Chernenko 1, 2, 3 , Alexander V. Astakhov 1, 2, 3 , Dmitry B. Eremin 3, 4, 5, 6 , Dmitry V. Pasyukov 1, 2, 3 , Alexey S. Kashin 3, 4, 5, 6 , Valentine P. Ananikov 1, 2, 3, 4, 5
Affiliation
|
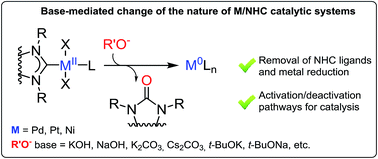
Numerous reactions are catalyzed by complexes of metals (M) with N-heterocyclic carbene (NHC) ligands, typically in the presence of oxygen bases, which significantly shape the performance. It is generally accepted that bases are required for either substrate activation (exemplified by transmetallation in the Suzuki cross-coupling), or HX capture (e.g. in a variety of C–C and C-heteroatom couplings, the Heck reaction, C–H functionalization, heterocyclizations, etc.). This study gives insights into the behavior of M(II)/NHC (M = Pd, Pt, Ni) complexes in solution under the action of bases conventionally engaged in catalysis (KOH, NaOH, t-BuOK, Cs2CO3, K2CO3, etc.). A previously unaddressed transformation of M(II)/NHC complexes under conditions of typical base-mediated M/NHC catalyzed reactions is disclosed. Pd(II) and Pt(II) complexes widely used in catalysis react with the bases to give M(0) species and 2(5)-oxo-substituted azoles via an O–NHC coupling mechanism. Ni(NHC)2X2 complexes hydrolyze in the presence of aqueous potassium hydroxide, and undergo the same O–NHC coupling to give azolones and metallic nickel under the action of t-BuOK under anhydrous conditions. The study reveals a new role of NHC ligands as intramolecular reducing agents for the transformation of M(II) into “ligandless” M(0) species. This demonstrates that the disclosed base-mediated O–NHC coupling reaction is integrated into the catalytic M/NHC systems and can define the mechanism of catalysis (molecular M/NHC vs. “NHC-free” cocktail-type catalysis). A proposed mechanism of the revealed transformation includes NHC-OR reductive elimination, as implied by a series of mechanistic studies including 18O labeling experiments.
中文翻译:

揭示了碱在催化系统活化/失活中的不寻常作用:M / NHC催化中的O–NHC偶联†
金属(M)与N-杂环卡宾(NHC)配体的络合物通常会在明显影响性能的氧碱的存在下催化许多反应。人们普遍认为,底物激活(例如铃木交叉偶联中的金属转移)或HX捕获(例如,在各种C–C和C–杂原子耦合,Heck反应,C–H功能化中)都需要碱,杂环化等)。这项研究提供了对M(II)/ NHC(M = Pd,Pt,Ni)配合物在溶液中的行为的见解,该配合物在常规参与催化的碱(KOH,NaOH,t- BuOK,Cs 2 CO 3,K)的作用下2一氧化碳3,等等)。公开了在典型的碱介导的M / NHC催化的反应条件下M( II)/ NHC络合物的先前未解决的转化。在催化中广泛使用的Pd( II)和Pt( II)配合物通过O–NHC偶联机理与碱反应生成M(0)物种和2(5)-氧代取代的唑。Ni(NHC) 2 X 2络合物在氢氧化钾水溶液存在下水解,并经过相同的O–NHC偶联,在t的作用下得到偶氮酮和金属镍-BuOK在无水条件下。这项研究揭示了NHC配体作为分子内还原剂将M(II)转变为“无配体” M(0)物种的新作用。这表明所公开的碱介导的O-NHC偶联反应已整合到催化M / NHC系统中,并且可以定义催化机理(分子M / NHC与“不含NHC”的鸡尾酒型催化)。揭示的转化的拟议机制包括NHC-OR还原消除,这是一系列机械研究(包括18 O标记实验)所暗示的。
更新日期:2018-06-06
中文翻译:

揭示了碱在催化系统活化/失活中的不寻常作用:M / NHC催化中的O–NHC偶联†
金属(M)与N-杂环卡宾(NHC)配体的络合物通常会在明显影响性能的氧碱的存在下催化许多反应。人们普遍认为,底物激活(例如铃木交叉偶联中的金属转移)或HX捕获(例如,在各种C–C和C–杂原子耦合,Heck反应,C–H功能化中)都需要碱,杂环化等)。这项研究提供了对M(II)/ NHC(M = Pd,Pt,Ni)配合物在溶液中的行为的见解,该配合物在常规参与催化的碱(KOH,NaOH,t- BuOK,Cs 2 CO 3,K)的作用下2一氧化碳3,等等)。公开了在典型的碱介导的M / NHC催化的反应条件下M( II)/ NHC络合物的先前未解决的转化。在催化中广泛使用的Pd( II)和Pt( II)配合物通过O–NHC偶联机理与碱反应生成M(0)物种和2(5)-氧代取代的唑。Ni(NHC) 2 X 2络合物在氢氧化钾水溶液存在下水解,并经过相同的O–NHC偶联,在t的作用下得到偶氮酮和金属镍-BuOK在无水条件下。这项研究揭示了NHC配体作为分子内还原剂将M(II)转变为“无配体” M(0)物种的新作用。这表明所公开的碱介导的O-NHC偶联反应已整合到催化M / NHC系统中,并且可以定义催化机理(分子M / NHC与“不含NHC”的鸡尾酒型催化)。揭示的转化的拟议机制包括NHC-OR还原消除,这是一系列机械研究(包括18 O标记实验)所暗示的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号