当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular Dynamics Study on the Structure and Dynamics of NaCl Solution Transport in the Nanometer Channel of CASH Gel
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2018-06-05 00:00:00 , DOI: 10.1021/acssuschemeng.8b02126 Dongshuai Hou 1 , Tao Li 1 , Pan Wang 1
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2018-06-05 00:00:00 , DOI: 10.1021/acssuschemeng.8b02126 Dongshuai Hou 1 , Tao Li 1 , Pan Wang 1
Affiliation
|
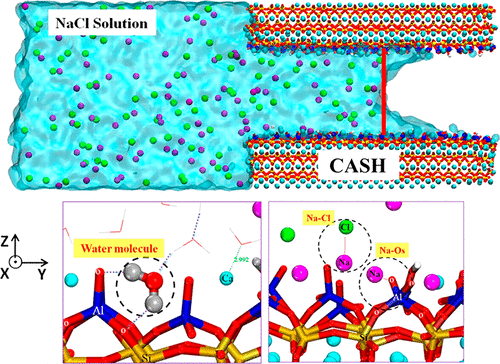
The transport of water molecules and ions in the nanopores of calcium aluminosilicate hydrate (CASH) influences the durability and sustainability of environmentally friendly cement-based materials with industrial waste substitution. In this study, molecular dynamics was utilized to study aqueous NaCl solution capillary transport through the calcium silicate hydrate (CSH) and calcium aluminosilicate hydrate (CASH) gel pore with pore size of 3.2 nm. The invading depth for the NaCl solution advancing frontier with meniscus shape follows a parabolic relation as a function of time, consistent with the classic Lucas–Washburn equation in capillary adsorption theory. As compared with the solution transport in the CSH pore, both water molecules and ions migrate more slowly in the gel pore of CASH, and sodium ions accumulate in the entrance region of the gel pore. The incorporation of Al atoms in the silicate substrate resists the ingress of ions and water. The Al–Si substitution on the CASH interface enhances the charge negativity of solid oxygen atoms, which polarizes the dipole moment of surface water molecules to a larger extent, strengthens the interfacial hydrogen bond, and elongates the residence time of water near the aluminate substrate. In addition, the silicate–aluminate chains in the CASH substrate provide plenty of oxygen sites to associate with the sodium ions by forming a stable Na–OS bond, immobilizing the cations deeply in the vacancy region of the aluminate–silicate channel. The inner sphere adsorption of Na ions on the CASH surface further contributes to the secondary outer sphere adsorption of the Cl ions by forming the Na–Cl ionic pairs. Hopefully, the transport and adsorption mechanism of the ions and water in the CASH gel can help guide the cementitious material substituted by Al-rich industry waste with sustainability and durability.
中文翻译:

NaCl溶液在CASH凝胶纳米通道中迁移的结构和动力学的分子动力学研究
水分子和离子在铝硅酸钙水合物(CASH)纳米孔中的运输影响了以工业废料替代的环保水泥基材料的耐久性和可持续性。在这项研究中,利用分子动力学研究了NaCl水溶液通过硅酸钙水合物(CSH)和硅铝酸钙水合物(CASH)凝胶孔的毛细管传输,孔径为3.2 nm。弯月面形状的NaCl溶液推进边界的侵入深度随时间呈抛物线关系,这与毛细管吸附理论中的经典Lucas-Washburn方程一致。与溶液在CSH孔中的迁移相比,水分子和离子在CASH的凝胶孔中迁移的速度更慢,钠离子积聚在凝胶孔的入口区域。在硅酸盐衬底中掺入Al原子可阻止离子和水的进入。CASH界面上的Al-Si取代增强了固体氧原子的电荷负性,使表面水分子的偶极矩极化更大程度,增强了界面氢键,并延长了水在铝酸盐基质附近的停留时间。此外,CASH基质中的硅酸盐-铝酸盐链通过形成稳定的Na-O提供了充足的氧位与钠离子缔合 它使表面水分子的偶极矩极化更大程度,增强了界面氢键,并延长了水在铝酸盐基质附近的停留时间。此外,CASH基质中的硅酸盐-铝酸盐链通过形成稳定的Na-O提供了充足的氧位与钠离子缔合 它使表面水分子的偶极矩极化更大程度,增强了界面氢键,并延长了水在铝酸盐基质附近的停留时间。此外,CASH基质中的硅酸盐-铝酸盐链通过形成稳定的Na-O提供了充足的氧位与钠离子缔合S键,将阳离子深深地固定在铝酸盐-硅酸盐通道的空位区域。Na离子在CASH表面上的内球吸附通过形成Na–Cl离子对进一步促进了Cl离子的第二外球吸附。希望,CASH凝胶中离子和水的传输和吸附机制可以帮助以可持续性和耐用性引导被富含Al的工业废料替代的胶凝材料。
更新日期:2018-06-05
中文翻译:

NaCl溶液在CASH凝胶纳米通道中迁移的结构和动力学的分子动力学研究
水分子和离子在铝硅酸钙水合物(CASH)纳米孔中的运输影响了以工业废料替代的环保水泥基材料的耐久性和可持续性。在这项研究中,利用分子动力学研究了NaCl水溶液通过硅酸钙水合物(CSH)和硅铝酸钙水合物(CASH)凝胶孔的毛细管传输,孔径为3.2 nm。弯月面形状的NaCl溶液推进边界的侵入深度随时间呈抛物线关系,这与毛细管吸附理论中的经典Lucas-Washburn方程一致。与溶液在CSH孔中的迁移相比,水分子和离子在CASH的凝胶孔中迁移的速度更慢,钠离子积聚在凝胶孔的入口区域。在硅酸盐衬底中掺入Al原子可阻止离子和水的进入。CASH界面上的Al-Si取代增强了固体氧原子的电荷负性,使表面水分子的偶极矩极化更大程度,增强了界面氢键,并延长了水在铝酸盐基质附近的停留时间。此外,CASH基质中的硅酸盐-铝酸盐链通过形成稳定的Na-O提供了充足的氧位与钠离子缔合 它使表面水分子的偶极矩极化更大程度,增强了界面氢键,并延长了水在铝酸盐基质附近的停留时间。此外,CASH基质中的硅酸盐-铝酸盐链通过形成稳定的Na-O提供了充足的氧位与钠离子缔合 它使表面水分子的偶极矩极化更大程度,增强了界面氢键,并延长了水在铝酸盐基质附近的停留时间。此外,CASH基质中的硅酸盐-铝酸盐链通过形成稳定的Na-O提供了充足的氧位与钠离子缔合S键,将阳离子深深地固定在铝酸盐-硅酸盐通道的空位区域。Na离子在CASH表面上的内球吸附通过形成Na–Cl离子对进一步促进了Cl离子的第二外球吸附。希望,CASH凝胶中离子和水的传输和吸附机制可以帮助以可持续性和耐用性引导被富含Al的工业废料替代的胶凝材料。


























 京公网安备 11010802027423号
京公网安备 11010802027423号