当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A one-pot process for the enantioselective synthesis of tetrahydroquinolines and tetrahydroisoquinolines via asymmetric reductive amination (ARA)†
Chemical Communications ( IF 4.9 ) Pub Date : 2018-06-06 00:00:00 , DOI: 10.1039/c8cc03586e Tao Yang 1, 2, 3, 4 , Qin Yin 1, 2, 3, 4 , Guoxian Gu 1, 2, 3, 4 , Xumu Zhang 1, 2, 3, 4
Chemical Communications ( IF 4.9 ) Pub Date : 2018-06-06 00:00:00 , DOI: 10.1039/c8cc03586e Tao Yang 1, 2, 3, 4 , Qin Yin 1, 2, 3, 4 , Guoxian Gu 1, 2, 3, 4 , Xumu Zhang 1, 2, 3, 4
Affiliation
|
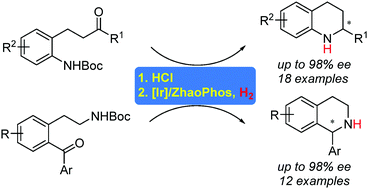
Asymmetric reductive amination for the synthesis of both chiral tetrahydroquinolines (THQs) and tetrahydroisoquinolines (THIQs) has been realized with an Ir/ZhaoPhos catalytic system via a one-pot N-Boc deprotection/intramolecular asymmetric reductive amination (ARA) sequence. Control experiments reveal that HCl plays a vital role to the success of this transformation. The HCl acid assists the removal of the N-Boc protecting group and also provides chloride ions to interact with the thiourea moiety in ZhaoPhos, thus leading to excellent reaction enantiocontrol.
中文翻译:

一锅法通过不对称还原胺化(ARA)对映选择性合成四氢喹啉和四氢异喹啉†
Ir / ZhaoPhos催化体系通过一锅N - Boc脱保护/分子内不对称还原胺化(ARA)序列实现了用于合成手性四氢喹啉(THQs)和四氢异喹啉(THIQs)的不对称还原胺化。对照实验表明,HCl对这种转化的成功起着至关重要的作用。HCl酸有助于除去N- Boc保护基,还提供氯离子与ZhaoPhos中的硫脲部分相互作用,从而实现出色的反应对映控制。
更新日期:2018-06-06
中文翻译:

一锅法通过不对称还原胺化(ARA)对映选择性合成四氢喹啉和四氢异喹啉†
Ir / ZhaoPhos催化体系通过一锅N - Boc脱保护/分子内不对称还原胺化(ARA)序列实现了用于合成手性四氢喹啉(THQs)和四氢异喹啉(THIQs)的不对称还原胺化。对照实验表明,HCl对这种转化的成功起着至关重要的作用。HCl酸有助于除去N- Boc保护基,还提供氯离子与ZhaoPhos中的硫脲部分相互作用,从而实现出色的反应对映控制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号