当前位置:
X-MOL 学术
›
J. Anal. Appl. Pyrol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic mechanism of sulfuric acid in cellulose pyrolysis: A combined experimental and computational investigation
Journal of Analytical and Applied Pyrolysis ( IF 6 ) Pub Date : 2018-09-01 , DOI: 10.1016/j.jaap.2018.06.007 Bin Hu , Qiang Lu , Yu-ting Wu , Zhen-xi Zhang , Min-shu Cui , Ding-jia Liu , Chang-qing Dong , Yong-ping Yang
Journal of Analytical and Applied Pyrolysis ( IF 6 ) Pub Date : 2018-09-01 , DOI: 10.1016/j.jaap.2018.06.007 Bin Hu , Qiang Lu , Yu-ting Wu , Zhen-xi Zhang , Min-shu Cui , Ding-jia Liu , Chang-qing Dong , Yong-ping Yang
|
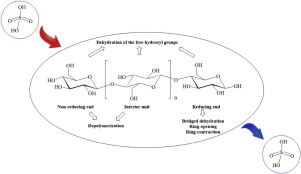
Abstract Sulfuric acid (H2SO4) is widely used as a strong acid catalyst in biomass pyrolysis process, and exhibits prominent catalytic effects on the pyrolytic reactions and product distribution. In this study, the fundamental catalytic mechanism of H2SO4 on cellulose pyrolysis process was investigated via combined experimental and computational methods. Both thermographic analysis (TGA) and pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS) experiments were performed to reveal the pyrolytic characteristics and product distribution in the H2SO4-catalyzed pyrolysis of cellulose. In addition, quantum chemistry methods were employed to build the reaction models and investigate the major H2SO4-assisted reactions in initial cellulose pyrolysis process, i.e., depolymerization of the cellulose chain, bridged dehydration, ring-opening, ring-contraction and dehydration of the free hydroxyl groups. The results indicate that the H2SO4-assisted depolymerization via C1-O1 bond scission and bridged dehydration reactions take place in a two-step mechanism involving a sulfate ester intermediate. While other reactions occur via a one-step mechanism involving only hydroxyl group or both hydroxyl and sulfonic groups of H2SO4. The activation energies of above reactions are all decreased by adding H2SO4, and thus lowering the degradation temperature of cellulose. Among all initial pyrolytic reactions, dehydration reactions which are inconspicuous in the non-catalytic process, become very important in the catalytic process, because their activation energies are decreased dramatically by adding H2SO4 (from 322.7 to 372.1 to 152.2–237.7 kJ/mol). The promoting effect on the dehydration reactions plays a vital role in the increased char yield and significant change of organic volatile product distribution. Through the promoted dehydration reactions, unsaturated structures of cellulose are formed, which is favorable for the formation of certain dehydrated products at the expense of depolymerized and ring scission products.
中文翻译:

硫酸在纤维素热解中的催化机理:结合实验和计算研究
摘要 硫酸(H2SO4)广泛用作生物质热解过程中的强酸催化剂,对热解反应和产物分布具有显着的催化作用。在这项研究中,通过结合实验和计算方法研究了 H2SO4 对纤维素热解过程的基本催化机制。进行了热解分析 (TGA) 和热解-气相色谱/质谱 (Py-GC/MS) 实验以揭示 H2SO4 催化的纤维素热解中的热解特性和产物分布。此外,采用量子化学方法建立了反应模型,研究了纤维素初始热解过程中主要的 H2SO4 辅助反应,即纤维素链的解聚、桥连脱水、开环、游离羟基的缩环和脱水。结果表明,通过 C1-O1 键断裂和桥接脱水反应的 H2SO4 辅助解聚发生在涉及硫酸酯中间体的两步机制中。而其他反应通过一步机制发生,仅涉及 H2SO4 的羟基或羟基和磺酸基。上述反应的活化能均因加入H2SO4而降低,从而降低纤维素的降解温度。在所有初始热解反应中,在非催化过程中不显眼的脱水反应在催化过程中变得非常重要,因为它们的活化能通过加入 H2SO4(从 322.7 到 372.1 到 152.2-237.7 kJ/mol)急剧下降。对脱水反应的促进作用在提高焦炭产率和显着改变有机挥发性产物分布方面起着至关重要的作用。通过促进脱水反应,形成纤维素的不饱和结构,这有利于某些脱水产物的形成,但以解聚和裂环产物为代价。
更新日期:2018-09-01
中文翻译:

硫酸在纤维素热解中的催化机理:结合实验和计算研究
摘要 硫酸(H2SO4)广泛用作生物质热解过程中的强酸催化剂,对热解反应和产物分布具有显着的催化作用。在这项研究中,通过结合实验和计算方法研究了 H2SO4 对纤维素热解过程的基本催化机制。进行了热解分析 (TGA) 和热解-气相色谱/质谱 (Py-GC/MS) 实验以揭示 H2SO4 催化的纤维素热解中的热解特性和产物分布。此外,采用量子化学方法建立了反应模型,研究了纤维素初始热解过程中主要的 H2SO4 辅助反应,即纤维素链的解聚、桥连脱水、开环、游离羟基的缩环和脱水。结果表明,通过 C1-O1 键断裂和桥接脱水反应的 H2SO4 辅助解聚发生在涉及硫酸酯中间体的两步机制中。而其他反应通过一步机制发生,仅涉及 H2SO4 的羟基或羟基和磺酸基。上述反应的活化能均因加入H2SO4而降低,从而降低纤维素的降解温度。在所有初始热解反应中,在非催化过程中不显眼的脱水反应在催化过程中变得非常重要,因为它们的活化能通过加入 H2SO4(从 322.7 到 372.1 到 152.2-237.7 kJ/mol)急剧下降。对脱水反应的促进作用在提高焦炭产率和显着改变有机挥发性产物分布方面起着至关重要的作用。通过促进脱水反应,形成纤维素的不饱和结构,这有利于某些脱水产物的形成,但以解聚和裂环产物为代价。



























 京公网安备 11010802027423号
京公网安备 11010802027423号