当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic Mechanism of DNA Polymerases: Contributions of Conformational Dynamics and a Third Divalent Metal Ion
Chemical Reviews ( IF 62.1 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acs.chemrev.7b00685 Austin T. Raper 1 , Andrew J. Reed 1 , Zucai Suo 1
Chemical Reviews ( IF 62.1 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acs.chemrev.7b00685 Austin T. Raper 1 , Andrew J. Reed 1 , Zucai Suo 1
Affiliation
|
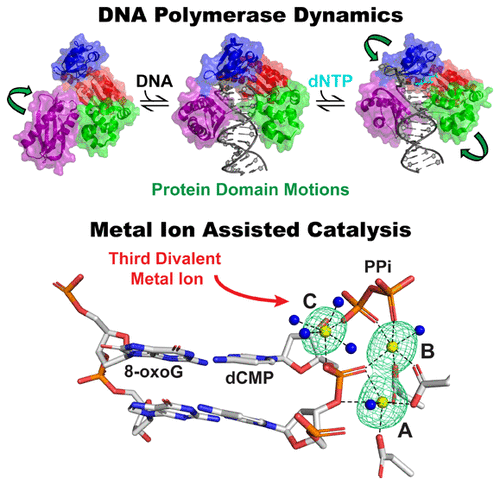
Faithful transmission and maintenance of genetic material is primarily fulfilled by DNA polymerases. During DNA replication, these enzymes catalyze incorporation of deoxynucleotides into a DNA primer strand based on Watson–Crick complementarity to the DNA template strand. Through the years, research on DNA polymerases from every family and reverse transcriptases has revealed structural and functional similarities, including a conserved domain architecture and purported two-metal-ion mechanism for nucleotidyltransfer. However, it is equally clear that DNA polymerases possess distinct differences that often prescribe a particular cellular role. Indeed, a unified kinetic mechanism to explain all aspects of DNA polymerase catalysis, including DNA binding, nucleotide binding and incorporation, and metal-ion-assisted nucleotidyltransfer (i.e., chemistry), has been difficult to define. In particular, the contributions of enzyme conformational dynamics to several mechanistic steps and their implications for replication fidelity are complex. Moreover, recent time-resolved X-ray crystallographic studies of DNA polymerases have uncovered a third divalent metal ion present during DNA synthesis, the function of which is currently unclear and debated within the field. In this review, we survey past and current literature describing the structures and kinetic mechanisms of DNA polymerases from each family to explore every major mechanistic step while emphasizing the impact of enzyme conformational dynamics on DNA synthesis and replication fidelity. This also includes brief insight into the structural and kinetic techniques utilized to study DNA polymerases and RTs. Furthermore, we present the evidence for the two-metal-ion mechanism for DNA polymerase catalysis prior to interpreting the recent structural findings describing a third divalent metal ion. We conclude by discussing the diversity of DNA polymerase mechanisms and suggest future characterization of the third divalent metal ion to dissect its role in DNA polymerase catalysis.
中文翻译:

DNA聚合酶的动力学机制:构象动力学和第三价金属离子的贡献。
遗传物质的忠实传输和维持主要由DNA聚合酶完成。在DNA复制过程中,这些酶基于与DNA模板链的Watson-Crick互补性,催化脱氧核苷酸掺入DNA引物链中。多年来,对每个家族的DNA聚合酶和逆转录酶的研究揭示了结构和功能上的相似之处,包括保守的结构域结构和声称的双金属离子核苷酸转移机理。但是,同样清楚的是,DNA聚合酶具有通常规定特定细胞作用的明显差异。实际上,有一个统一的动力学机制可以解释DNA聚合酶催化的所有方面,包括DNA结合,核苷酸结合和掺入以及金属离子辅助的核苷酸转移(即 化学),很难定义。特别地,酶构象动力学对几个机械步骤的贡献及其对复制保真度的影响是复杂的。此外,最近对DNA聚合酶的时间分辨X射线晶体学研究发现了DNA合成过程中存在的第三种二价金属离子,目前尚不清楚其功能,并且在本领域内尚有争议。在这篇综述中,我们调查了过去和现在的文献,这些文献描述了每个家族的DNA聚合酶的结构和动力学机制,以探索每个主要的机械步骤,同时强调了酶构象动力学对DNA合成和复制保真度的影响。这还包括对用于研究DNA聚合酶和RT的结构和动力学技术的简要见解。此外,在解释描述第三种二价金属离子的最新结构发现之前,我们提供了DNA聚合酶催化的两种金属离子机理的证据。我们通过讨论DNA聚合酶机制的多样性来得出结论,并提出了第三二价金属离子的未来表征,以剖析其在DNA聚合酶催化中的作用。
更新日期:2018-06-04
中文翻译:

DNA聚合酶的动力学机制:构象动力学和第三价金属离子的贡献。
遗传物质的忠实传输和维持主要由DNA聚合酶完成。在DNA复制过程中,这些酶基于与DNA模板链的Watson-Crick互补性,催化脱氧核苷酸掺入DNA引物链中。多年来,对每个家族的DNA聚合酶和逆转录酶的研究揭示了结构和功能上的相似之处,包括保守的结构域结构和声称的双金属离子核苷酸转移机理。但是,同样清楚的是,DNA聚合酶具有通常规定特定细胞作用的明显差异。实际上,有一个统一的动力学机制可以解释DNA聚合酶催化的所有方面,包括DNA结合,核苷酸结合和掺入以及金属离子辅助的核苷酸转移(即 化学),很难定义。特别地,酶构象动力学对几个机械步骤的贡献及其对复制保真度的影响是复杂的。此外,最近对DNA聚合酶的时间分辨X射线晶体学研究发现了DNA合成过程中存在的第三种二价金属离子,目前尚不清楚其功能,并且在本领域内尚有争议。在这篇综述中,我们调查了过去和现在的文献,这些文献描述了每个家族的DNA聚合酶的结构和动力学机制,以探索每个主要的机械步骤,同时强调了酶构象动力学对DNA合成和复制保真度的影响。这还包括对用于研究DNA聚合酶和RT的结构和动力学技术的简要见解。此外,在解释描述第三种二价金属离子的最新结构发现之前,我们提供了DNA聚合酶催化的两种金属离子机理的证据。我们通过讨论DNA聚合酶机制的多样性来得出结论,并提出了第三二价金属离子的未来表征,以剖析其在DNA聚合酶催化中的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号