当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Promoting Intermolecular C–N Bond Formation under the Auspices of Iodine(III)
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acs.accounts.8b00137 Kilian Muñiz 1, 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acs.accounts.8b00137 Kilian Muñiz 1, 2
Affiliation
|
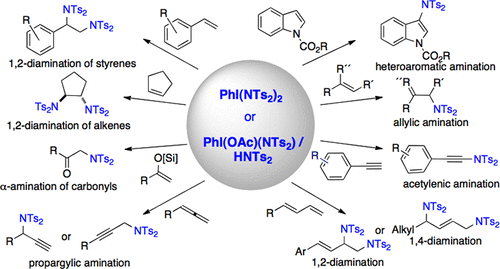
The quest for the development of new protocols that provide general conditions for oxidative carbon–nitrogen bond formation has grown over recent years. Within this context, due to feasibility and benignity considerations in biochemical sciences, reactions that rely on main group oxidants as the only promoters have received particular interest. We have recently found that simple protonolysis events enable the incorporation of nitrogenated groups of the bissulfonimide family into the coordination sphere of common iodine(III) complexes such as diacetoxy iodobenzene. The products of the type ArI(OAc)(NTs2) represent rare examples of iodine(III) compounds displaying reactive iodine–nitrogen single bonds. Further protonolysis furnishes the corresponding iodine(III) compounds ArI(NTs2)2 containing two defined iodine–nitrogen single bonds for unprecedented dual transfer of both nitrogenated groups. It is of great synthetic importance that these new compounds contain iodine–nitrogen entities, which upon dissociation in solution lead to electrophilic iodine centers and nucleophilic nitrogen groups. This has enabled the development of a body of conceptually new amination reactions, which do not rely on conventional electrophilic nitrogen reagents but rather employ iodine(III) as an electrophilic activator and bissulfonimides as the source of subsequent nucleophilic amination. Additional diversification arises from the ambident nature of bissulfonimines enabling oxygenation pathways.
中文翻译:

在碘的主持下促进分子间C–N键的形成(III)
近年来,对开发为氧化碳-氮键形成提供一般条件的新方案的要求不断增长。在这种情况下,由于生化科学中的可行性和良性考虑,依赖主族氧化剂作为唯一促进剂的反应受到了特别的关注。我们最近发现,简单的质子分解事件使双磺酰亚胺家族的硝化基团并入普通碘(III)配合物(如二乙酰氧基碘苯)的配位范围内。ArI(OAc)(NTs 2)类型的产物代表了具有反应性碘-氮单键的碘(III)化合物的稀有实例。进一步的质子分解提供相应的碘(III)化合物ArI(NTs 2)2包含两个已定义的碘-氮单键,可实现两个硝化基团的前所未有的双重转移。这些新化合物包含碘-氮实体,这在合成中具有极其重要的意义,该实体在溶液中解离后会导致亲电子碘中心和亲核氮基团。这使得能够开发一系列概念上新的胺化反应,该反应不依赖于常规的亲电子氮试剂,而是使用碘(III)作为亲电子活化剂,并使用双磺酰亚胺作为后续亲核胺化的来源。双硫亚胺具有充氧途径的环境性质使其具有额外的多样化优势。
更新日期:2018-06-04
中文翻译:

在碘的主持下促进分子间C–N键的形成(III)
近年来,对开发为氧化碳-氮键形成提供一般条件的新方案的要求不断增长。在这种情况下,由于生化科学中的可行性和良性考虑,依赖主族氧化剂作为唯一促进剂的反应受到了特别的关注。我们最近发现,简单的质子分解事件使双磺酰亚胺家族的硝化基团并入普通碘(III)配合物(如二乙酰氧基碘苯)的配位范围内。ArI(OAc)(NTs 2)类型的产物代表了具有反应性碘-氮单键的碘(III)化合物的稀有实例。进一步的质子分解提供相应的碘(III)化合物ArI(NTs 2)2包含两个已定义的碘-氮单键,可实现两个硝化基团的前所未有的双重转移。这些新化合物包含碘-氮实体,这在合成中具有极其重要的意义,该实体在溶液中解离后会导致亲电子碘中心和亲核氮基团。这使得能够开发一系列概念上新的胺化反应,该反应不依赖于常规的亲电子氮试剂,而是使用碘(III)作为亲电子活化剂,并使用双磺酰亚胺作为后续亲核胺化的来源。双硫亚胺具有充氧途径的环境性质使其具有额外的多样化优势。



























 京公网安备 11010802027423号
京公网安备 11010802027423号